2.6.5.6 Phenolic Ether Volatile Oils
A good number of volatile oils essentially contain phenolic ethers which attribute powerful aromatic odour and flavour. Because of their distinct characteristic aroma they are used extensively as pharmaceutical aids, perfumery and confectionery. A few typical examples of phenolic ether volatile oils are, namely: Anethole; Safrole; Myristicin; Apiole; Cineole and Ascaridole.
General Properties of Phenolic Ether Volatile Oils There are certain characteristic general properties of phenolic ether volatile oils that help in their identifications:
1. They are very stable neutral compounds which are sparingly water-soluble. They do not react with alkalies.
2. Phenolic ethers, in general, yield the corresponding phenols on treatment with HBr or HCl.
3. They form crystalline derivatives on account of various reactions, such as: bromination, nitration and oxidation.
4. Phenolic ethers give rise to the formation of sulphonamides in a two-step reaction depicted below:
Step 1: It react with chloro sulphonic acid to yield the corresponding sulphonyl chloride together with a molecule each of hydrochloric acid and sulphuric acid.
Step 2: The resulting sulphonyl chloride (Step 1) on reaction with ammonium carbonate gives rise to the desired sulphonamide and a mole each of ammonium chloride, carbon dioxide and water.
These chemical constituents shall be discussed individually in the sections that follows:
A. Anethole (Synonym Anise camphor, Monasirup)
Chemical Structure 1-Methoxy-4-(1-propenyl) benzene.
It has a monohydric phenolic ether function.
Occurrence It is the chief constituent of anise (anise fruit, aniseed) i.e., the dried ripe fruits of Pimpinella anisum Linn' (Family: Umbelliferae); star anise (star anise fruit, Chinese anise i.e., the dried ripe fruits of Illicium verum Hoop (Family: Magnoliaceae); and fennel (fennel fruits finnocchio), i.e., the dried ripe fruits of Foeniculum vulgare Mill (Family: Apiaceae). It is also found in Ocimum basilicum L. (Family: Lamiaceae)-Sweet Basil, Garden Basil; Pinus elliottii Engelm. (Family: Abiataceae)-Slash Pine; Sassafras albidum (Nutt.) Nees (Family: Lauraceae)-sassafras; and Syzygium aromaticum (L.) Merr & Perry (Family: Myrtaceae)-cloves, clavos.
Isolation It may be isolated from the volatile oils by first subjecting the oil to fractionation and then cooling the corresponding fraction to a very low temperature and recrystallization. However, it may also be obtained directly from the anethole-rich oils, such as: oil of anise, oil of fennel by simply chilling it to – 30°C in a deep freezer. Commercially, anethole may be synthesized in its purest form from anisole as shown below:
Anisole on reacting with propionaldehyde in the presence of HCl and H3PO4 yields an intermediate anisole-p-(1-chloropropane) which finally with pyridine yields anethole.
Characteristic Features It exists in two isomeric forms namely: trans-and cis-isomer, having physical parameters as stated below:
It is a white crystalline substance with an intense sweet odour. It possesses a characteristic taste similar to anise fruit. It is practically soluble in most organic solvents but insoluble in water.
Formation of ‘Photoanethole’ (or p, p′-dimethoxystilbene) Anethole on exposure to air (oxygen), light or heat undergoes structural modifications to yield photoanethole which is a viscid yellow coloured mass having a disagreeable taste and odour with a poor solubility in solvents. Perhaps the conversion of anethole to photoanethole lakes place via the formation of anisaldehyde as given below:
Identification
1. Anethole undergoes oxidation with K2Cr2O7in two steps; first step-yields anisaldehyde (paramethoxy benzaldehyde), and second step-gives rise to para-methoxy benzoic acid (mp 184°C) as depicted below:
2. It gets condensed with maleic anhydride to yield a condensation product having mp 310°C as shown below:
3. It gives rise to the formation of nitroso derivative having mp 126°C.
Uses
1. It is used as a flavouring agent in perfumery particularly for soap and dentifrices.
2. It is also employed as a pharmaceutical and (flavour).
3. It finds its application as an imbedding material in microscopy.
4. It is employed as a flavouring agent in alcholic, non-aleoholic beverages and confectionaries.
5. It is used as a sensitizer in bleaching colours in colour photography.
B. Safrole
Chemical Structure 5-(2-Propenyl)-1, 3-benzodioxole; 4-allyl-1, 2-methylenedioxybenzene.
Occurrence It is the constituent of a number of volatile oils, notably of sassafras i.e., the dried dark of the roots of Sassafras albidum Nees, belonging to the family Lauraceae, in which it is present to the extent of 75%.
It is extensively found in a variety of other plant sources, namely: Acorus calamus L., Araceae (sweet flag, flagroot, calamus); Angelica polymorpha Max., Apiaceae (dong quai); Cananga odorata (Lam.) Hook. f. & Thoms., Annonaceae (cananga, ylang-ylang); Cinnamomum comphora (L.) J.S. Presl., Lauraceae (camphor, hon-sho); Illicum verum Hook. f. Magnoliaceae (Star-anise,Chinese anise); Myristica fragrans Houtt. Myristicaceae (mace, nutmeg); Ocimum basilicum L. Lamiaceae (sweet basil, garden basil); Piper nigrum L. Piperaceae (black pepper); Theobroma
cacao L. Sterculiaceae (chocolate, cocoa, cacao); Umbellularia california (Hook. and Arn.) Nutt. (California bay, California sassafras, (California laurel).
Isolation Safrole may be isolated from the oil of sassafras, comphor oil and oil of star-anise and also the safrole-rich fraction of the oil to about –10 to –15°C. It may also be isolated by subjecting the above safrole containing oils to fractional distillation under reduced pressure, chilling the fraction and finally crystallization.
Characteristic Features It is colourless or slightly yellow liquid having a specific sassafras odour. Its physical properties are: d20 1.096, mp ~ 11°C, bp 232-234°C and n20D1.5383. It is insoluble in water, very soluble in alcohol and freely miscible with ether and chloroform. It undergoes isomerization on being heated with alkalies to yield isosafrole as shown below:
Identification
1. Bromination: Safrole on bromination yields the corresponding pentabromosafrole (mp
169-170°C).
2. Oxidation: Safrole on oxidation with K2Cr2O7 and dilute H2SO4(6 N) gives rise to the aldehyde derivative piperonol as shown below:
3. Colour test: Both safrole and isosafrole on treatment with concentrated sulphuric acid instantly produces an intense red colouration.
Uses
1. It is widely used as a flavouring agent for a variety of products, such as: beverages,
pharmaceuticals chewing gums, toothpastes, in perfumery and scenting soaps.
2. It is also used in denaturing fats in soap manufacturing process.
3. It is mostly employed for the conversion to isosafrole and the manufacture of heliotropin.
C. Myristicin
Chemical Structure 4-(Methoxy)-6-(2-propenyl)-1, 3-benzodioxole.
Occurrence The aromatic ether is extracted from nutmeg, mace, French parsley, carrots and dill oils.
The botanical sources of myristicin are as follows: Anethum graveolens L. (Apiaciae) (Dil, Dill Seed, Garden Dill); Daucus Carota subsp. Sativus (Hoffm.) Arcang [Apiaceae] (Cultivated carrot,
Queen Anne’s Lace (Wild)); Myristica fragrans Houtt. [Myristaceae] (Mace, Nutmeg); Petroselinum crispum (Mill) Nym. [Apiaceae] (Parsley); Piper nigrum L. [Piperaceae] (Black Pepper); Sassafras albidum (Nutt.) Nees [Lauraceae] (Sassafras).
Isolation The rich source of volatile oil containing myristicin is subjected to fractional distillation under reduced pressure when the latter is collected as a colourless oily liquid.
Characteristic Features It is an oily liquid having a characteristic aromatic odour. It does not congeal at low-temperature.
Myristicin on being treated with either metallic sodium or boiled with alcoholic KOH undergoes isomerism to yield isomyristicin as given below:
i.e., the allyl group in the former gets converted to the propenyl group in the latter.
It has the following physical parameters:
bp40 173°C; n20D1.54032; d2020 1.1437.
Identification
1. On oxidation with KMnO4 it gives rise to two products, namely:
(a) Myristicin aldehyde (mp 130°C); and
(b) Myristinic acid (mp 208-210°C).
2. On interaction with bromine it yields the corresponding dibromoderivative having mp 130°C.
Uses It is used as a flavouring agent in food products and confectioneries.
D. Apiole
Synonym Dill; Dill apiole; Parsley comphor.
Chemical Structure 4,5-Dimethoxy-6-(2-propenyl)-1, 3-benzodioxole.
Occurrence It occurs abundantly in dill oil Anethum graveolus L., belonging to the natural order Umbelliferae. It is also found in the Parsley seed oil Petroselinum crispum (Mill.) Nym. (Family: Apiaceae). The volatile oil of Sassafras albidum (Nutt.) Nees (Family: Lauraceae) contains apiole.
Isolation It is obtained by chilling the volatile oil to a very low temperature in a deep-freezer and finally recrystallizing it either from ethanol or petroleum ether (mp 29.5°C).
Characteristic Features Apiole crystallises usually in the shape of long colourless needles with a faint specific odour of Parsley. Its physical parameters are: mp 29.5°C, bp 285°C; n17D1.5305; d15151.1598. It is practically insoluble in water, but soluble in ethanol, ether and in fatty oils.
Apiole on boiling with alcoholic KOH undergoes isomerisation to yield isoapiole (mp 55-56°C) whereby the allyl group in the former gets isomerized to the propenyl function in the latter as given below:
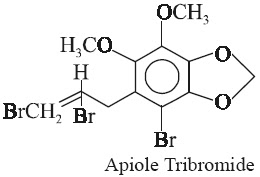
Apiole on treatment with bromine yields a monobromide (mp 51°C), a dibromide (mp 75°C) and also a tribromide (mp 120°C) as depicted below:
On oxidation with KMnO4 both apiole and isoapiole yield the corresponding apioaldehyde and apiolic acid.
Identification
1. It may be identified by forming its bromoderivatives as stated above having a specific melting point.
2. It may also be identified by preparing its oxidative products with KMnO4, such as: opioaldeyde (mp 102°C) and apiolic acid (mp 173°C).
Uses
1. It exerts a synergistic activity with insecticides.
2. Dill is frequently employed as an aromatic stimulant, carminative and flavouring agent.
3. Dill oil is an important ingredient of ‘Gripe Water’ which is given to infants to relieve them from flatulence.
E. Cineole
Synonyms Eucalyptol; Cajeputol.
Chemical Structure 1, 8-Epoxy-p-menthane.
Occurrence It is the chief constituent of oil of eucalyptus obtained from the leaves of Eucalyptus globulus Labill (Family: Myrtaceae) and other species of Eucalyptus. It also occurs largely in a variety of plants, namely: Acorus calamus L., (Araceae); Aloysia triphylla Britton (Family: Verbenaceae)-Lemon Verbena; Artemisia vulgaris L., (Family: Asteraceae)-Mugwort, Carline Thistle; Chamaemelum nobile (L.) All (Family: Asteraceae)-Roman Camomile, English Camomile, Camomile, Cinnamomum verum J.S. Presl (Family: Lauraceae)-Ceylon Cinnamon; Crocus sativus
L., (Family: Iridaceae)-Saffron, Saffron crocus; Croton eleutheria Sw. (Family: Euphorbiaceae-Cascarilla; Illicium verum Hook. f. (Family: Magnoliaceae)-Star-Anise, Chinese Anise; Juniperus communis L. (Family: Cupressaceae)-Common Juniper; Juniperus sabina L. (Family Cupressaceae)-Sabine, Savin; Laurus nobilis L., (Family: Lauraceae)-Bay, Grecian Laurel, Green Bay; Melaleuca
leucadenron L. (Family: Myrtaceae)-Cajeput; Pimenta diocia (L.) Merr. (Family: Myrtaceae)-Allspice, Jamaica Pepper, Clove Pepper; Rosmarinus officinalis L. (Family: Lamiaceae)-Rosemary; Salvia sclarea L., Family: Lamiaceae)-Clary, Muscatel Sage; Tanecetum vulgare L., (Family: Asteraceae)-Tansy; Umbellularia californica (Hook and Arn.) Nutt.-California Bay, California Laurel, California Sassafras.
Isolation Cineole may be isolated from Eucalyptus oil, which contains this ingredient to the extent of 80% by any one of the following four methods, namely:
Method I: Fractional Distillation. It may be obtained from fractional distillation under vacuo and
the colourless liquid is collected over powdered anhydrous sodium sulphate. The clear oily substance is obtained finally in the pure crystalline form by chilling it (mp + 1.5°C).
Method II: Addition Products with Halogen Acids (HCl, HBr). It forms addition compounds with HCl and HBr as: C10H18O.HCl and C10H18O . HBr, from which the pure cincole may be regenerated conveniently.
Method III: Addition Product with Resorcinol. It forms an addition compound with 50% (w/v) solution of pure resorcinol as [(C10H18O)2.C6H6O2] having mp 80-85°C, from which cineole may be regenerated easily.
Note This reaction may eater for the separation of cineole from essential oils having a high cineole content (more than 50-60%), otherwise the volatile oil must first be fractionated.
Method IV: Addition Product with Phosphoric Acid. Cineole readily forms addition product with phosphoric acid as: [C10H18O.H3PO4] having mp 84°C, that may be decomposed by hot water.
Note This method is also utilized for the estimation of cineole in volatile oils in v/v percentage.
Characteristic Features It is a colourless liquid having a camphor-like odour. It possesses a spicy and cooling taste. Its physical characteristics are: d2525 0.921-0.923, bp 176-177°C, mp + 1.5°C, n20D 1.455-1.460, flash point (closed-up) 48°C. It is almost insoluble in water but miscible with alcohol,
chloroform, ether, glacial acetic acid and oils.
Cineole forms addition compounds with resorcinol and phosphoric acid, that are found to be fairly stable, having mp 80-85°C and 80°C respectively.
It is not attacked by ordinary reducing agents, such as: glucose etc.
Identification
1. Cineole may be characterized by a host of derivatives/addition compounds obtained from pure chemical substances, for instance: halogen acids, resorcinol, phosphoric acid, orthocresol etc.
2. Microchemical Test of Cineole: A drop of pure cineole or a drop of Eucalyptus oil or a few drops of an alcoholic extract of eucalyptus leaf, is made to react with a drop of 5% (w/v) solution of hydroquinone on a microscopic slide and subsequently examined under a low-power microscope one may observe either colourless prisms or rhomboid crystals.
However, an identical treatment with a 50% (w/v) solution of resorcinol gives rise to beautiful leaf-like crystals.
Uses
1. It is used quite extensively in pharmaceutical preparations both meant for internal and external utilities, such as:
Internal usage—as a stimulating expectorant in cases of chromic bronchitis
External usage—as a mild antiseptic, anaesthetic in cases of inflammatory conditions.
2. It is also employed in room-sprays, hand lotions and all types of cosmetic formulations.
3. It is invariably used as a pharmaceutical aid i.e., flavouring agent.
F. Ascaridole (Synonym Ascarisin)
Chemical Structure 1, 4-Peroxido-p-menthene-2. It is an organic peroxide which constitutes 60-80% of oil of chenopodium. It is the only naturally occurring terpenoid peroxide.
Occurrence Ascaridiole is the major constituent (65-70%) in the chenopodium oil, i.e., a volatile oil, obtained by the steam distillation from the fresh flowing and fruiting plants (except roots) of the botanical species Chenopodium ambrosioides var anthelminticum Linn., belonging to the family Chenopodiaceae.
Isolation It is isolated by the repeated fractional distillation of the volatile oil of chenopodium (American wormseed oil) under vacuo and collecting the fraction boiling at 95-98°C.
Characteristic Features Ascaridiole is a viscid yellow oily liquid having a very peculiar and most disagreable odour and flavour. It is highly unstable and is prone to explode when either subjected to heat or when treated with organic acids, e.g., acetic acid; and with inorganic acids, e.g., sulphuric, nitric; hydrochloric and phosphoric acids. It being a peroxide liberates I2 from KI in acetic acid
solution. It is soluble in hexane, pentane, ethanol, toluene, benzene and castor oil. Its physical characteristics are: mp + 3.3°C; bp0.2 39-40°C; [α]20D 0.00; d2041.0103;
Prepared Synthetically Ascaridiole may be synthesized from µ-terpinene by treatment with oxygen, chlorophyll and light as given below:
Identification As ascaridiole does not produce any crystalline derivative, therefore, it is usually characterized by the help of the following two specific reactions:
1. Formation of cis-1:4-Terpin [C10H18(OH)2]: Ascaridiole on reduction with H2 and Pd as a catalyst gives rise to the formation of cis-1, 4-terpin as follows:
The resulting cis-1, 4-terpin is optically inactive and also it is not identical with 1, 8-terpin, although the two compounds have similar melting points i.e., 116–117°C.
2. Formation of Ascaridole Glycol: Ascaridole upon oxidation with FeSO4 yield chiefly a ‘glycol’ which is not steam-volatile viz., ascaridole glycol. Consequently, this glycol may be further characterized by the formation of its monobenzoate (mp 136-137°C) and dibenzoate derivatives (mp 116.5°C).
Uses
1. It has been used as an anthelmintic (Nematodes).
2. It is also employed for eliminating hookworms and roundworms.
3. It is used most frequently in large number of medical and veterinary formulations.
Note Estimation of Ascaridole* Ascaridole may be determined quantitatively by the method described in the Extra Pharmacopoea, which is developed by Cocking and Hymas and based upon the oxidizing property of the ‘peroxide function’ exclusively present in it on the strongly acidified solution of KI (with HCl and glacial acetic acid). Thus, the liberated I2 is titrated with sodium thiosulphate (Na2S2O3) using freshly prepared starch solution as an indicator (colour change from blue to colourless) under the specified experimental parameters.
Precautions
1. Addition of oxygenated constituents must be avoided in the assay procedure that may give rise to eroneous results.
2. As the liberated iodine is capable of being absorbed by unsaturated components present in the volatile oil, it is absolutely necessary to carry out the assay at low temperature so as to maintain such secondary reactions at the lowest level.