2.1 Alkaloids Derived from Amination Reactions
It has been duly established that the larger section of alkaloids are virtually derived from amino acid precursors by the help of certain specific processes that essentially introduce into the final structure not only a N-atom but also an amino acid carbon skeleton or a major part of it. However, a good number of alkaloids do not essentially conform with this analogy. They are usually synthesized primarily from non-amino acid precusors having the N-atom inserted into the structure at a comparatively latter stage. Interestingly, such structures are predominantly based on both steroidal and terpenoid skeletons. Besides, a few comparatively simpler alkaloids also appear to be derived exclusively with the help of similar late amination processes. An extensive and intensive studies on certain alkaloids it has been observed that the N-atom is specifically donated from an amino acid source through a transamination reaction using an appropriate ketone or aldehyde.
2.1.1 Acetate-Derived Alkaloids
Socrates was made to drink the decoction of the Hemlock plant and died soonafter. Thus, the poison present in it is really too dangerous for herbal administration by the uninitiated. The Hemlock plant is comprised of several potent alkaloids, such as: coniine, γ-coniceine, conhydrine, N-methyl conine and pseudoconhydrine. These alkaloids shall now be discussed as under:
A. Coniine
Synonyms Cicutine, Conicine.
Biological Sources It is obtained from the unripe, fully grown dried fruits of Conium maculatum
L. (Umbelliferae).
It also occurs in the plant Aethusa cynapium L. (Apiaceae) (Fool’s Parsley); Cicuta maculata L.
(Apiaceae) (Water Hemlock).
Chemical Structure
(S)-2-Propylpiperidine. It occurs naturally as the (S)-(+)- isomer.
Isolation Coniine may be isolated by adopting the various following steps, namely:
(i) The powdered unripe, fully grown dried fruits of hemlock are mixed with a dilute solution of KOH and then subjected to stream distillation. The distillate is collected and neutrallized carefully with dilute HCl and evaporated to dryness preferably under vacuum.
(ii) The residue obtained as stated in (i) above is extracted with alcohol, filtered and the alcohol evaporated to dryness under vacuum. The alcohol helps in extracting the alkaloidal salts that are dissolved in water; it is then rendered alkaline either with diluted KOH solution or with dilute NH4OH and finally extracted with ether successively.
(iii) The ether from the combined ethereal layer is evaporated completely, when an oily liquid consisting of the free bases remains in the residue.
(iv) Finally, the residue is subjected to fractional distillation in a current of H2-gas when the alkaloids could be broadly separated and a mixture containing coniine and γ-coniceine shall pars over as the first fraction at 171-172°C. These two alkaloids are consequently made to their corresponding hydrochloride salts, evaporated to dryness and extracted with acetone.
Thus, coniine hydrochloride would be separated as an insoluble product, while the γ –coniceine may be recovered by evaporating acetone under vacuum.
Note: Coniine enjoys the unique distinction of being the First Alkaloid produced synthetically.
Characteristic Features
(i) It is a colourless alkaline liquid.
(ii) It darkens and polymerizes on being exposed to air and light.
(iii) It has a mousy odour.
(iv) Its physical parameters are as follows: mp ~ – 2°C; bp 166-166.5°C; bp20 65-66°C. d420 0. 844-0.848; - nD23 1.4505; α D25 +8.40C (c = 4.0 in CHCl3); α D23 +14.60(heat) pKa = 3.1.
(v) It is steam volatile.
(vi) Solubility: 1 ml dissolves in 90 ml of water, less soluble in hot water. The base dissolves in about 25% water at room temperature. It is found to be soluble in alcohol, ether, acetone, benzene, amyl alcohol, and slightly soluble in chloroform.
Identification Tests
(i) It readily forms the corresponding hydrobromide (C8H17N.HBr), obtained as prisms, mp 211°C, 1 g dissolves in 2 ml water, 3 ml alcohol, and soluble freely in ether and chloroform.
(ii) Its hydrochloride (C8H17N . HCl) forms rhomboids, mp 221°C, freely soluble in water, alcohol and chloroform.
(iii) It gives a red colouration with sodium nitroprusside slowly, which on addition of acetaldehyde changes to violet or blue.
Caution It exhibits potential symptoms of over exposure as: weakness, drowsiness, parasthesias, ataxia, nausea, excessive salivation, and bradycardia followed by tachycardia.*
Uses Externally, the coniine salts are used as ointments and infrequently employed for their local analgesic action in the symptomatic relief of pruritis, hemorrhoids and fissures.
B. γ-Coniceine
Biological Source It is obtained from the seeds of Conium maculatum L. (Umbelliferae).
Chemical Structure
2, 3, 4, 5-Tetrahydro-6-propylpyridine.
Characteristic Features
(i) It is a colourless liquid alkaloid.
(ii) It possesses a distinct mousy odour.
(iii) It is steam volatile.
(iv) Its physical parameters are: bp 171°C; d415 0.8753; nD16 1.4661.
(v) It is slightly soluble in water, but freely soluble in ethanol, chloroform and ether.
Identification Test
(i) -Coniceine when subjected to reduction, it gives rise to a racemic mixture of dl-coniine.
(ii) It forms γ-coniceine hydrochloride (C8H15N.HCl) which gives hygroscopic crystals from ether mp 143°C.
C. Conhydrine
Biological Source It is obtained from the seeds of Conium maculatum L. (Umbelliferae).
Chemical Structure
[R-(R*, S*)-a-Ethyl-2-piperidine methanol.
Characteristic Features
(i) The crystals obtained from ether has mp 121°C, bp 226°C and [α]D + 10°C.
(ii) It is slightly soluble in water, but easily soluble in ethanol, ether and chloroform.
D. N-Methylconiine
Biological Source It is same as for (C) above.
Chemical Structure
1-Methyl-2-propylpiperidine.
Isolation The d-form is stated to occur in Hemlock in small quantities, while the l-form may be isolated from residues left in the preparation of coniine by crystallization of the hydrobromides.
Characteristic Features The physical characteristic features of dl, d- and l-forms are given below:
E. Pseudoconhydrine
Biological Source Its biological source is same as for (A) through (D) above.
Chemical Structure
(3S-trans)-6-Propyl-3-piperidinol.
Characteristic Features
(i) It gives hygroscopic needles from absolute ether.
(ii) Its mp stands at 106°C, whereas its monohydrate, scales, gives mp 60°C from moist ether.
(iii) Its physical parameters are: bp 236°C ;[α]D20 +110 (c = 10 in alcohol); pK (18°C): 3.70
(iv) It is soluble in water and
Identification Tests It readily forms the hydrochloride salt (C8H17NO.HCl) as the crystals from ethanol having mp 213ºC.
Biosynthesis of γ-Coniceine and Coniine A fatty acid precursor octanoic acid (capric acid) is employed, which is subsequently transformed into the ketoaldehyde through successive oxidation and reduction steps. The resulting ketoaldehyde acts as a substrate for a transamination reaction, the amino moiety is derived from L-alanine. The ultimate transformation lead to the formation of imine giving the heterocyclic ring present in g-coniceine, and then reduction the coniine as shown below:
2.1.2 Phenylalanine-Derived Alkaloids
It has been observed that the aromatic amino acid L-tyrosine is not only a common but also an extremely vital precursor of alkaloids; whereas, L-phenylalanine is found to be much less frequently employed, and normally it specifically contributes carbon atoms only, such as: C6C1, C6C2or C6C3 units, without making available a N-atom from its amino function e.g., as in the biosynthesis of colchicine and lobeline.
The various typical examples of phenylalanine-derived alkaloids are: ephedrine, norpseudoephedrine (cathine) and capsaicin, which shall be described hereunder:
A. Ephedrine
Biological Source It occurs in the dried young stems of the Chinese wonder drug Ma Huang, Emhedra vulgaris, Ephedra sinica Stapf., Ephedra equisetina Bunge belonging to family Ginetaceae, and also in several other Ephedra species. This is also found in Ephedra geradiana Wall ex. Stapf. (Ephedraceae) (Pakistani Ephedra). There are two most important forage ephedras in the United States, namely: E. nevadensis and E. viridis. The former are is E. nevadensis S. Wats (Ephedraceae) and known as Mormon Tea and Nevada Jointfir.
Chemical Structure
α-[1-(Methylamino)ethyl] benzene methanol (C10H15HO).
Isolation Ephedrine usually exists singly in Ephedra sinica (1-3%) and E. equisetina (2%).
However, it occurs in association with ~| -Ephedrine (i.e., pseudoephedrine) in E. vulgaris.
However, the ephedrine and pseudoephedrine may be extracted conveniently from the dried young stems of the plant material by adopting the ‘general procedures for alkaloid extraction’ (section 1.7.3), by the help of successive benzene and dilute HCl extractions.
Preparation Ephedrine may be prepared by two methods, namely:
(i) Fermentation method, and
(ii) Synthetic method.
(a) Fermentation Method: It can be prepared commercially by fermenting a mixture of molasses** and benzaldehyde. The reaction product i.e., methyl benzyl alcohol ketone i.e., C6H5-CH(OH)COCH3, a keto-alcohol is subsequently mixed with a solution of methyl amine and freshly prepared H2-gas is made to pass though it. Thus, we have:
(b) Synthetic Method: Manske et al.*** (1929) synthesized (±)-Ephedrine by the catalytic reduction of 1-phenylpropane-1, 2-dione (or benzoylacetyl) in the presence of methylamine in methanol solution as given below:
Stereochemistry Since the ephedrine molecule contains two dissimilar chiral centres, four optically active isomers (or two pairs of enantiomers) are possible theoretically. Freudenberg (1932) put forward the following configurations of ephedrine and ψ-ephedrine (mp 118°C, [α]D ± 51.2°) are as follows:
Foder et al. (1949, 1950) confirmed that the ephedrine has the erythro-configuration, and yephedrine the threo-configuration as stated below:
The carbobenzoxy derivative of nor-ψ-ephedrine undergoes intramolecular rearrangement to the O-derivative in an acidic medium. In case, nor-ψ-ephedrine possesses the threo-configuration, then this ultimately gives rise to the favourable trans-orientation of the phenyl and methyl groups in the cyclic intermediate i.e., the steric repulsions are at a bear minimum level. Likewise, the nor-ephedrine shall, therefore, exhibit essentially the crythroconfiguration; and it was further revealed that its corresponding N-carbobenzoxy derivative does not undergo any molecular rearrangement whatsoever in an acidic environment to produce the O-derivative. Therefore, one may infer that the steric repulsions that would take place between the phenyl and methyl groups in Foder et al. (1949, 1950) confirmed that the ephedrine has the erythro-configuration, and ψ-ephedrine the threo-configuration as stated below: the cyclic intermediate is evidently too high to allow its subsequent formation. Thus, it is absolutely possible, on this basis, to differentiate and distinguish between the stereoisomers of ephedrine and ψ-ephedrine.
Characteristic Features The characteristic features of various forms of ephedrine and its salts are as stated under:
Special Features
(i) Ephedrine does not yield a precipitate with Mayer’s Reagent except in concentrated solution.
(ii) Ephedrine in chloroform solution after long standing or on evaporation usually forms ephedrine hydrochloride and phosgene.
(iii) Both ephedrine and pseudoephedrine are fairly stable to heat and when heated at 100°C for several hours does not undergo any decomposition.
(iv) Ephedrine hydrochloride on being heated with 25% HCl gets partially converted to pseudoephedrine; and this conversion is reversible and soon attains on equilibrium.
Identification Tests
(i) Colour Test: Dissolve 0.1 g ephedrine in 1 ml water with the addition of a few drops of dilute HCl. Add to it two drops of CuSO4 solution (5% w/v) followed by a few-drops of NaOH (1N) solution when a reddish colour is obtained. Add to it 2-3 ml of ether and shake vigorously, the ethereal layer becomes purple and the aqueous layer turns blue.
(ii) Formation of Ephedrine Hydrochloride: Dissolve 0.2-0.3g of ephedrine in 35 ml of chloroform in a stoppered test tube and shake vigorously. Allow it to stand for 12 hours and evaporate the chloroform, when crystals of ephedrine HCl are obtained, and
(iii) Formation of Benzaldehyde Odour: Take 0.05 g of ephedrine in a small porcelain dish and triturate it with a few crystals of pure potassium ferricyanide, [K3Fe(CN)6], add a few drops of water and heat on a water-bath, it gives rise to a distinct odour of benzaldehyde.
Biosynthesis of Ephedrine Alkaloids Interestingly, phenylalanine and ephedrine not only have the same carbon and nitrogen atoms but also have the same arrangement of C and N-atoms i.e., the skeleton of atoms. Noticeably, L-phenylalanine is a precursor, possessing only seven carbons, a C6C1 fragment, gets actually incorporated. It has been observed that phenylalanine undergoes metabolism, probably via cinnamic acid to benzoic acid; and this perhaps in the form of its coenzyme–A ester, which is acylated with pyruvic acid and undergoes decarboxylation during the addition as shown below.
A thiamine PP-mediated mechanism is put forward for the formation of the diketone, and a transamination reaction shall give rise to cathinone. Further reduction of the carbonyl moiety from either face yields the diastereomeric norephedrine or norpseudoephedrine (Cathine). Ultimately,
N-methylation would give rise to ephedrine or pseudoephedrine.
Uses
1. The l-ephedrine is extensively used as a bronchodilator.
2. The d-psendoephedrine is employed widely as a decongestant.
B. Norpseudoephedrine
Synonyms Cathine; Katine; Nor-y-ephedrine.
Biological Sources It occurs naturally as the D-threo-form in the leaves of the khat plant, Catha edulis Forsk. (Celastraceae), which is widely found as an evergreen shrub native to Southern Arabia and Ethiopia. It is also found in relatively smaller amounts in the South American tree Maytenus krukovii A.C. Smith (Celastraceae); and in the mother liquors obtained from Ma Huang after the recovery of ephedrine.
Chemical Structure
(R*, R*)-α-(1-Aminoethyl)-benzenemethanol.
Isolation It is isolated from the plant material as described under (A) in this section.
Characteristic Features The various physical parameters of different forms of norpseudoephedrine are summarized below:
Uses
1. It is widely employed as an anorexic.
2. It is also used in the optical resolution of externally compensated acids.
C. Capsaicin
Synonyms Axsain; Mioton; Zostrix.
Biological Source It is the pungent principle obtained in the fruit of various species of Capsicum, viz., Capsicum annum L. (Solanaceae) (Chilli, Sweet Peppers, Paprika).
Chemical Structure
(E)-N-[4-Hydroxy-3-methoxyphenyl)-methyl]-8-methyl-6-noenamide. It is phenolic in nature.
Isolation The capsicum fruits are crushed and extracted with either hot acetone or ethanol by using the method of percolation. The solvent i.e., hot acetone or ethanol is evaporated under vacuum.
The residue is extracted once again with successive quantities of warm acetone or ethanol until and unless the marc is completely free from any pungent principles. It contains approximately not less than 8% of capsaicin.
Characteristic Features
1. Capsaicin gives a distinct burning taste even when diluted to the extent of one part in one million parts of water. However, its pungency is destroyed by oxidation.
2. It is obtained as monoclinic, rectangular plates, scales from petroleum ether, having mp 65°C.
3. It has bp 0.01 210-220°C (air-bath temperature).
4. It has uv maximum: 227, 281 nm (€ 7000, 2500).
5. It is freely soluble in ether, benzene, chloroform; slightly soluble in CS2; and practically in soluble in water.
Identification Tests
1. An alcoholic solution of capsaicin gives rise to a distinct bluish green colour upon adding a few drops of FeCl3 solution (0.5% w/v).
2. When capsaicin is dissolved in a few drops of concentrated H2SO4 and a few crystals of sucrose is added, it yields a violet colour after a few hours.
Uses
1. It is used as a topical analgesic.
2. It is often employed as a tool in neurobiological research.
3. It is used in creams to counter neuralgia caused by herpes infections and in other pain-relieving formulations.
Biosynthesis of Capsaicin The aromatic fragment of the capsaicin molecule is derived solely from phenylalanine through chemical entities, viz., ferulic acid and vanillin. The later compound, an aldehyde, is actually the substrate for transamination to yield vanillylamine. However, the acid part of the resulting amide structure is of polypeptide origin having essentially a branched-chain fatty acyl-CoA which is produced by chain extension of isobutyryl-CoA. The aforesaid source of reactions are as given under:
---------------------------------------------------------------
* Gosselin et al. Eds. Clinical Toxicology of Commercial Products, Williams and Wilkins, Baltimore, 5th ed., Sec-
II., pp. 249-250 (1984).
** Molasses: A thick brown viscous liquid obtained as a by product of ‘Sugar Industry’ containg 8-10% cane sugar.
*** Manske and Holmes (eds). The Alkaloids, Academic Press. N. York. Vol. 1 (1950)
2.1.3 Terpenoid Alkaloids
A plethora of alkaloids solely based on mono-, sesqui-, di-, and tri-terpenoid skeletons have been isolated and characterized. However, logistic and scientific information (s) with regard to their actual formation in nature is more or less sparse. It has been observed that the monoterpene alkaloids are derived from the structurally related iridoid materials, wherein the O-atom in the heterocyclic ring is replaced by a N-containing ring as depicted below.
A few typical examples of the terpenoid alkaloids are, namely: aconine and actinitine, which shall be discussed in the sections that follow:
A. Aconine
Biological Source Aconine is the hydrolyzed product of aconitine which is obtained from the dried roots of Aconitum napellus Linn. (Ranunculaceae) and other aconites. A. napellus in also known as aconite, blue rocket and monkshood. Usually it contains upto 0.6% of the total alkaloids of aconite, of which approximately one third is the alkaloid aconitine.
Chemical Structure (1α, 3α, 6α, 14α, 15α, 16α)-20-Ethyl-1, 6-16-trimethoxy-4-(methoxymethyl) aconitane-3, 8, 13, 14, 15-pentol.
Isolation The alkaloid aconitine is subjected to hydrolysis which yields benzoyl aconine and acetic acid. The resulting benzoyl aconine is further hydrolyzed to yield aconine and benzoic acid.
Aconine being very soluble in water may be separated easily from the less water-soluble by product, i.e., benzoic acid.
Characteristic Features
(i) It is an amorphous powder with a bitter taste.
(ii) It has mp 132°C, [α]D+ 23° and pKa 9.52.
(iii) It is extremely soluble in water, alcohol; moderately soluble in chloroform and slightly soluble in benzene. It is practically insoluble in ether and petroleum ether.
Identification Tests It forms two distinct derivatives as given below:
(a) Aconine Hydrochloride Dihydrate (C25H42ClNO9.2H2O): It is obtained as crystals having mp 175-176°C and [α]D–8°.
(b) Aconine Hydrobromide Sesquihydrate (C25H42BrNO9 .1½ H2O]: It is obtained as crystals from water with mp 225°C.
Uses
1. It is used in the treatment of neuralgia, sciatica, rheumatism and inflammation.
2. It is employed occasionally as analgesic and cardiac depressant.
B. Aconitine
Biological Source The bolanical source is the same as described under (A) above.
Chemical Structure
(1α, 3α, 6α, 14α, 15α, 16(β)-20-Ethyl-1, 6,-16-trimethoxy-4-(methoxymethyl) aconitane-3, 8, 13, 14, 15,-pentol 8-acetate 14-benzoate (C34H47NO11).
Characteristic Features
1. It occurs as hexagonal plates having mp 204°C.
2. Its pKa value stands at 5.88.
3. Its specific rotation [α]D+ 17.3° (Chloroform).
4. It is slightly soluble in petroleum ether; but 1 g dissolves in 2 ml chloroform, 7 ml benzene, 28ml absolute ethanol, 50 ml ether, 3300 ml water.
Identification Tests Aconitine forms specific salts with HBr and HNO3 having the following physical parameters.
(a) Aconitine Hydrobromide Hemipentahydrate (C34H47O11.HBr.2½ H2O): The hexagonal tablets mp 200-207°C and the dried substance mp 115-120°C. Its crystals obtained from ethanol and ether with ½ H2O has mp 206-207°C. Its specific rotation [α]D – 30.9°.
(b) Aconitine Nitrate (C34H47NO11.HNO3): The crystals have mp about 200°C (decomposes), [α]D20 -350(c = 2 in H2O).
Uses
1. It is exclusively used in producing heart arrythmia in experimental animals.
2. It has also been used topically in neuralgia.
Biosynthesis of Aconitine-Type Alkaloids Aconite is particularly regarded as extremely toxic, due to the presence of aconitine, and closely related C19nonditerpenoid alkaloids. It has been observed that the species of Delphinium accumulate diterpenoid alkaloids, for instance: atisine, which proved to be much less toxic when compared to aconitine. A vivid close resemblance of their structural relationship to diterpenes, such as: ent-kaurene, of course, little experimental evidence is available.
From the above course of reactions it appears quite feasible that:
(a) A pre-ent-kaurene carbocation usually undergoes Wagner-Meerwein Rearrangements,
(b) The atisine-skeleton is produced subsequently by incorporating an N–CH2–CH2–O fragment (e.g., from 2-aminoethanol) to form the resulting heterocyclic rings,
(c) The aconitine-skeleton is perhaps formed from the atisine-skeleton by further modifications as stated above,
(d) A rearrangement process converts two fused 6-membered rings into a (7 + 5)-membered bicyclic system, and
(e) One carbon from the exocyclic double bond is eliminated.
2.1.4 Steroidal Alkaloids
In general, the steroidal alkaloids represent an important class of alkaloids that essentially afford a close structural relationship to sterols i.e., they contain a perhydro-1, 2-cyclopentanophenanthrene nucleus. Interestingly, these group of alkaloids invariably occur in the plant kingdom as glycosidal combination with carbohydrate moieties.
The steroidal alkaloids may be broadly classified into two major groups, namely:
(a) Solanum Alkaloids, and
(b) Veratrum Alkaloids.
These two class of alkaloids shall now be discussed in an elaborated fashion hereunder:
A. Solanum Alkaloids
A good number of plants belonging to the natural order Solanaceae have been found to accumulate favourably several steroidal alkaloids based on a C27 cholestane skeleton, such as: solasodine, tomatidine, solanidine. These alkaloids usually occur in a wide variety of the genus Solanum, for instance: Solanum laciniatum; S. dulcamara Linn.; S. nigrum Linn.; S. torvum Swartz.; S. lycopersicum Linn.; (Lycopersicon exculentum Mill); S. tuberosum; S. aviculare etc. The three above mentioned alkaloids normally occur naturally in the plant as their corresponding glycosides.
However, the two species of Solanum, namely: S. laciniatum and S. aviculare are considered to be a rich source of alkaloids (i.e., the aglycone moieties) that are employed exclusively as the starting materials for the synthesis of several hormones and adreno-cortical steroids.
The solanum alkaloids, stated above are essentially the nitrogen-analogues of steroidal saponins.
Unlike, their oxygen counterparts, all these N-containing alkaloids exhibit the same stereochemistry at C-25 (methyl being equatorial always), but C-22 isomers do exist, such as: solasodine and tomatidine.
The above cited three members of the solanum alkaloids shall be discussed as under:
A.1 Solasodine
Synonyms Solancarpidine; Solanidine-S; Purapuridine.
Biological Sources It is obtained from the fruits of Capsicum annuum L. (Solanaceae) (Chili,
Paprika, Sweet Peppers); shoots and berries of S. dulcamara L. (Solanaceae) (Bittersweet, Bitter
Nightshade, Felonwood); leaves of S. nigrum L. (Solanaceae) (Wonderberry, Black Nightshade,
Prairie Huckleberry).
Chemical Structure
(3b, 22a, 25R)-Spirosol-5-en-3-ol; (C27H43NO2).
Isolation It is obtained by the hydrolysis of solasonine which yields solasodine, L-rhamnose, Dgalactose and D-glucose respectively. It is the dehydrated product.
Characteristic Features
(ii) It has mp 200-202°C; [α]D25 -98o [c = 0.14 in methanol); [α]D –113° (CHCl3); pKb 6.30.
(iii) It is freely soluble in benzene, pyridine, and chloroform; moderately soluble in ethanol, methanol, and acetone; slightly soluble in water and practically insoluble in ether.
Identification Tests (for Solanum Alkaloids)
1. Dissolve 5-10 mg of the alkaloid in a few drops of hot amyl alcohol or ethanol and allow it cool gradually. The appearance of jelly-like product gives the characteristic test of the solanum alkaloids.
2. When a few mg of the alkaloids is treated with antimony trichloride solution in dry chloroform, it gives rise to a distinct red colouration.
3. The solanum alkaloids, in general, produces an instant red-violet colour with formaldehyde (HCHO) and sulphuric acid (H2SO4). This particular test is so distinct and sensitive that it is used for the quantitative estimation of these alkaloids colorimetrically.
Uses It is invariably used as a starting material for steroidal drugs.
---------------------------------------------
* GGPP = Geranylgeranyl diphosphate
A.2 Tomatidine
Biological Source It is obtained from the roots of Rutgers tomato plant [Lycopersicon esculentum Mill., cultivar. “Rutgers”] (Solanaceae) (Tomato).
Chemical Structure (3β, 5α, 22β, 25 S)-Spirosolan-3-ol; (C27H45NO2).
Characteristic Features
1. It is obtained as plates from ethyl acetate having mp 202-206°C.
2. It specific rotation [α ]D25 + 8o (chloroform).
Isolation It is obtained by the hydrolysis of tomatine to yield a molecule of tomatidine along with 2 moles of D-glucose, 1-mole of D-xylose and 1-mole of D-galactose as depicted below:
Identification Test Its hydrochloride derivative (C27H45NO2.HCl) is obtained as crystals from absolute ethanol having mp 265-270°C and [α ]D25 -5o (methanol).
A.3 Solanidine
Synonym Solatubine.
Biological Source The plant of Capsicum annuum L. (Solanaceae) (Chili, Peppers, Paprika) contains solanidine.
Chemical Structure
(3b)-Solanid-5 en-3-ol; (C27H43NO).
Isolation It is obtained by the hydrolysis of solanine which yields one mole each of L-Rhamnose, D-Galactose, and D-Glucose as shown below.
Characteristic Features
1. The long needles obtained from chloroform-methanol have a mp 218-219°C. It usually sublimes very close to its mp with slight decomposition.
2. It is specific rotation [α ]D21 -29o (c = 0.5 in CHCl3).
3. It is freely soluble in benzene, chloroform, slightly in methanol and ethanol; and almost insoluble in ether and water.
Identification Tests The same as described under A.1. earlier in this section. Besides, it has the following specific features for the corresponding derivatives, namely:
(a) Hydrochloride Derivative: (C27H43NO.HCl): Prisms from 80% alcohol and gets decomposed at 345°C.
(b) Methyliodide Derivative: (C27H43NO.CH3I): Crystals from 50% (v/v) ethanol and decomposes at 286°C.
(c) Acetylsolanidine Derivative: (C29H45NO2): Crystals obtained from ethanol having mp 208°C.
Biosynthesis of Solasodine, Tomatidine and Solanidine Like the sapogenins, the steroidal alkaloids are also derived from cholesterol, with suitable side-chain modification during the course of biochemical sequence of reactions as given under.
From the above biochemical sequence of reactions it is evident that:
(i) L-arginine seems to be used as a source for N-atom through amination via a substitution process upon 26-hydroxycholesterol,
(ii) Another substitution affords 26-amino-22-hydroxycholesterol to cyclize thereby forming a heterocyclic piperidine ring,
(iii) After 16β-hydroxylation, the secondary amine is oxidized to an imine, and the ultimate spirosystem may be envisaged by virtue of a nucleophilic addition of the 16β-hydroxyl on to theimine, and
(iv) This specific reaction, however, establishes the configurations, viz: 22R-as in the case of Solasodine, and 22S-as in the case of Tomatidine.
B. Veratrum Alkaloids The Veratrum alkaloids represent the most important and medicinally significant class of steroidal alkaloids. It is, however, pertinent to mention here that the basic ring systems present in the Veratrum alkaloids are not quite the same as seen in the usual steroidal nucleus, as present either in the cholesterol or in the aglycone residues of the cardiac glycosides
(A). Interestingly, one may observe in the structures of Veratrum alkaloids that the ring ‘C’ is a fivemembered ring while ring ‘D’ is a six-membered ring (B) which apparently is just the reverse of the pattern in the regular steroidal nucleus as depicted in next page.
Examples
(a) Alkamine portion of the ester alkaloids of Veratrum, viz., Protoverine, Veracevine, Germine.
(b) Alkamine aglycones of glycosidic veratrum alkaloids, viz., Veratramine.
In general, the majority of Veratrum alkaloids may be classified into two categories solely based on their characteristic structural features, namely:
(i) Cevaratrum alkaloids, and
(ii) Jeveratrum alkaloids
These two categories of Veratrum alkaloids shall now be discussed individually in the sections that follows:
B.1 Ceveratrum Alkaloids The important alkaloids belonging to this group of alkaloids are, namely: Protoveratrines; Veratridine, Cevadine, Germine etc., which shall be treated separately hereunder:
B.1.1 Protoveratrines
Biological Sources It is obtained from the rhizome of Veratrum album L. (Liliaceae) and Veratrum viride Ait. (Liliaceae) (American Hellebore).
However, the alkaloids present in the rhizomes of V. viride are placed in three groups, such as:
Group-‘A’: Alkamines (esters of the steroidal bases) with organic acids, including germidine, germitrine, most valued therapeutically; besides, cevadine, neogermitrine, neoprotoveratrine, protoveratrines and veratridine,
Group-‘B’: (Glycosides of the alkamines), mainly pseudojervine and veratrosine, and
Group-‘C’: (Alkamines), germine, jervine, rubijervine, and veratramine.
Chemical Structure
Isolation Protoveratrine A and B are usually extracted together and referred to as ‘protoveratrines’. About 2 kg of dried rhizomes of V. album is powdered and then extracted with benzene and ammonia. The total alkaloids are purified by extraction with acetic acid, re-extracted into benzene. The solvent is removed under vacuum, the residue is dissolved in ether from which the crystalline powder of the crude protoveratrines separates out. The crude product is recrystallized from alcohol-acetic acid and upon subsequent alkalinization of the solution with dilute ammonia. By this method one may obtain 8-10 g of protoveratrine powder from 8 kg of V. album rhizomes. Consequently, protaverine A and B may be separated by the help of counter current distribution of the “protaverine” between benzene and acetate buffer (pH 5.5) and ultimately subjected to column chromatography on acid aaluminium oxide (Al2O3).
Characteristic Features The characteristic features of the protoveratrines are as follows:
(i) The sternutative crystals obtained from ethanol have a slightly bitter taste.
(ii) It decomposes at 266-267°C.
(iii) Its specific rotation [α]D25 -38.6o (pyridine), and [α]D25-85o(C = 1.99 in chloroform).
(iv) It is soluble in chloroform, dilute aqueous acidic solutions and slightly soluble in ether. It is practically insoluble in water and petroleum ether.
However, the characteristic features of protoveratrine A and B are as stated below:
Uses
1. It is used as an antihypertensive agent which exerts its action through reflex inhibition of pressor
receptors in the heart and carotid sinus.
2. It also possesses emetic action.
3. It is used in the treatment of toxemia of pregnancy.
---------------------------------------------------
* Blount, J. Chem. Soc, 122, (1935); Vejdelek et. al. Chem. Listy 153, 33, (1956); Coll. Czech. Chem. Commun., 22, 98 (1957).
B.1.2 Veratridine
Biological Sources It is obtained from the seeds of Schoenocaulon officinale (Schelecht. And Cham.) A. Gray and also from the rhizome of Veratrum album L. (Liliaceae).
Chemical Structure (3β, 4α, 16β)-4, 9-Epoxycevane-3, 4, 12, 14, 16, 17, 20-heptol 3-(3, 4-dimethoxybenzoate) as given under.
Isolation Veratridine can be isolated as the commercial veratrine (mixture) i.e., the mixture of alkaloids cevadine, veratridine, cevadiline, sabadine and cevine obtained from the seeds of S. officinale stated above, as its sparingly soluble nitrate derivative.*
Characteristic Features
1. It is yellowish-white amorphous powder.
2. It tenaciously retains water.
3. It has mp 180°C after drying at 130°C.
4. Its specific rotation is [ α]D20 +8.0o (ethanol) and pKa 9.54 ± 0.02.
5. It is insoluble in water but slightly soluble in ether.
Identification Tests
1. It readily forms its nitrate derivative which is an amorphous powder and sparingly soluble in water.
2. Its sulphate salt is formed as its needles which happens to be very hygroscopic.
3. It readily forms its perchlorate derivative as long needles from water having mp 259-260°C (after drying at 120°C in Vacuo).
B.1.3 Cevadine
Synonym Veratrine
Biological Source It is obtained from the seeds of Schoenocaulon officinale (Schlecht and Cham.)
A. Gray (Sabadilla officinarum Brandt.) belonging to family Liliaceae.
Chemical Structure [3β(Z), 4α, 16β]-4, 9-Epoxycevane-3, 4, 12, 14, 16, 17, 20-heptol 3-(2-methyl-2-butenoate) as stated below.
1. It gives rise to flat needles from ether which decomposes at 213-214.5°C.
2. It has specific rotation [α] D20 +12.8o (C = 3.2 in ethanol).
3. Solubility: 1 g dissolves in 15 ml ether or ethanol and is very slightly soluble in wager.
Identification Tests
1. It forms aurichloride derivative which are obtained as fine yellow needles from ethanol that gets decomposed at 190°C.
2. It readily produces mercurichloride derivative (C32H49NO9.HCl.HgCl2) as silvery scales which decomposes at 172°C.
Caution Cevadine is extremely irritating locally particularly to the mucous membranes. Caution must be used in handling.
B.1.4 Germine
Biological Source Germine (an alkamine) is present in a plethora of polyester alkaloids that occur in Veratrum and Zygadenus species, such as: Veratrum viride Ait. (Liliaceae).
Chemical Structure (3α, 4α, 7α, 15α, 16β)-4, 9-Epoxycevane-3, 4, 7, 14, 15, 16, 20-heptol (C27H43NO8).
1. It is obtained as crystals from methanol mp 221.5-223 C°.
2. It has specific rotation [α]D25 +4.5o(95% ethanol) and [α]D16+23.1o (C = 1.13 in 10% acetic acid).
3. Solubility: It is soluble in chloroform, methanol, ethanol, acetone and water; and slightly soluble in ether.
Identification Tests It forms three different types of ‘acetates’ having specific characteristic features as stated below:
1. 3-Acetate derivative of Germine (C29H45NO9): It forms needles from ether having mp 219-221°C and [α]D23 +1.0o(C = 1.05 in pyridine).
2. 16-Acetate derivative of Germine (C29H45NO9): It forms crystals from chloroform having mp 225-227°C and [α]D23 -19o(C = 0.93 in pyridine).
3. 3, 4, 7, 15, 16-Pentaacetate derivative of Germine (C37H53NO13): It yields prisms from acetone + petroleum ether which decomposes at 285-287°C and [α]D23-65o(C = 0.65 in pyridine).
B.2 Jeveratrum Alkaloids The Jeveratrum group of alkaloids is usually represented by the structure of veratramine, jervine and pseudojervine etc., which essentially have the following salient features showing the points of difference in comparison to the Ceveratrum alkaloids: The three important members of this particular category of alkaloids shall be treated separately in sections that follows:
B.2.1 Veratramine
Biological Sources It is obtained in the rhizomes of Viratrum viride Ait. (Liliaceae) (American Hellebore); and also from Veratrum grandiflorum (Maxim.) Loes. F. (Liliaceae).
Chemical Structure The chemical structure of veratramine has also been referred to as azasteroid, wherein the N-atom is present is in one or more side chains.
(3β, 23β)-14, 15, 16, 17-Tetrahydro-veratraman-3, 23-diol (C27H39NO2).
Characteristic Features
1. It is obtained as crystals having mp 206-207°C.
2. It is slightly soluble in water, but soluble in ethanol and methanol.
Identification Tests
1. It forms a complex with digitonin (1:1) that has uvmas : 268 nm and [α ]D25 -71.8o (C = 1.21; [α ]D25 -70o (C = 1.56 in methanol).
2. Dihydroveratramine Derivative: The crystals of dihydroveratramine derivative has mp 192.5- 194°C ; [α]D25 +26o(C = 1.26 in acetic acid).
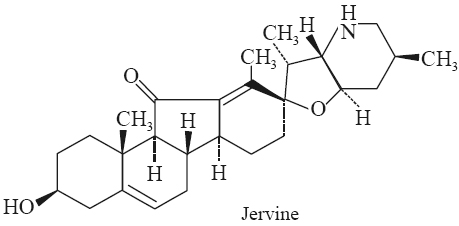
B.2.2 Jervine
Biological Sources It is obtained in the rhizomes of Veratrum grandiflorum (Maxim.) Loes F. Veratrum album L., and Veratrum viride Sol. (Liliaceae).
Chemical Structure
(3b, 23b)-17, 23-Epoxy-3-hydroxyveratraman-11-one (C27H39NO3).
Characteristic Features
1. The needles obtained from methanol and water has mp 243.5-244°C (Saito).
2. Its specific rotation [α]D20150 - ∞ (ethanol) (Saito); and [α]D20 -167.6o(chloroform) (Poethke).
3. It has uvmax : 250, 360 nm (€ 1500, 60).
Identification Tests
1. Diacetyljervine (C31H43NO5): The diacetyljervine has mp 173-175°C; [α]D – 112°; uvmax (ethanol): 250, 360 nm (€ 16400, 80).
2. Jervine Hydrochloride has mp 300-302°C.
B.2.3 Pseudojervine
Biological Sources It is obtained from the rhizomes of Veratrum viride Ait (Liliaceae) (American Hellebore); V. album L. (Liliaceae); and V. eschscholtzii Gray. (Liliaceae).
Chemical Structure It is the glucoside of jervine as given below.
(3β, 23β)-17, 23-Epoxy-3-(β-D-glucopyrnosyloxy) veratraman-11-one. (C33H49NO8).
Characteristic Features
1. It is obtained as lustrous leaflets having mp 300-301°C (dec.).
2. It specific rotation [α]D25 -133o (C = 0.48 in 1.3 ethanol-chloroform).
3. Solubility: It is soluble in benzene, chloroform; slightly soluble in ethanol and almost insoluble in ether.
Note: It is, however, pertinent to observe here that the Zygadenus species and the Schoenocaulon species appear to have only the Ceveratrum alkaloids and practically no Jeveratrum alkaloids. Interestingly, the large number of Veratrum species seem to contain both these type of steroidal alkaloids.




































![Veratramine [Jeveratrum Alkaloid] Veratramine [Jeveratrum Alkaloid]](https://blogger.googleusercontent.com/img/b/R29vZ2xl/AVvXsEhkLNYWM0MKtNHndWBkW24gT88npDpGG7LC5QRSV-yDNDfm5HhqZQ9CibQesiRWV_pAmPscq59uOOugEAO9It-KFjw19Tayb8I9ldaS8O8XjkS72ytaSYMIHsu3lj9GH9Tr1ma22iy7kw5s/s320/61-Veratramine.jpg)
![Cevadine [Ceveratrum Alkaloid] Cevadine [Ceveratrum Alkaloid]](https://blogger.googleusercontent.com/img/b/R29vZ2xl/AVvXsEgt7qI1LeCgtIErK50w3791zt8Npwuv6K8dh7BFJAcvjFW9kA9eenURwOynmzBBnwOH1lqfBog_b05CjYQZO9ubwTgltdrnvmPS5oGuQ_IssZR3o3mF1esod9JmBinTA3-2S3xYxXktUEp6/s1600/62-Cevadine.jpg)