1. Resin Acids
Synonyms Resinolic Acid.
The resin acids essentially contain a large portion of carboxylic acids and phenols. However, they occur both in the free state and as their respective esters. They are usually found to be soluble in aqueous solutions of the alkalies, thereby forming either soap like solutions or colloidal suspensions.
Resinates, i.e., the metallic salts of these acids find their extensive usage in the manufacture of inferior varities of soaps and varnishes.
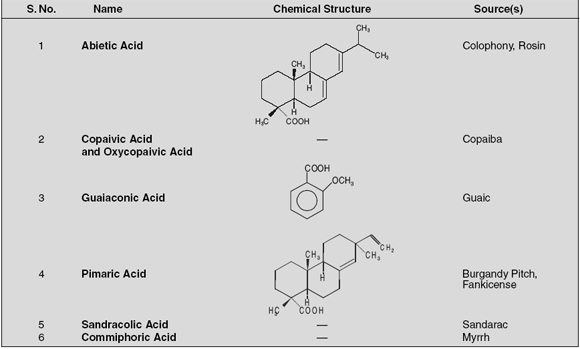
A few typical examples of resin acids are enumerated below:
Out of all the six commonly found resin acids Abietic Acid shall be discussed here under:
Abietic Acid (Synonym Sylvic Acid)
Chemical Structure 13-Isopropylpodocarpa-7, 13-dien-15-oic acid; (C20 H30O2).
It is a tricyclic diterpene embedded with four isoprene units. It is studded with four methyl moieties and a carboxylic acid function. Besides, it also has two double bonds one each in ring-Band ring-C of the phenanthrene nucleus.
Preparation It is a widely available organic acid, prepared by the isomerization of rosin.* It may also be synthesized from dehydroabietic acid.**
The commercial grade of abietic acid is normally obtained by heating either rosin alone or with mineral acids. The product thus achieved may be glassy or partly crystalline in nature. It is usually of yellow colour and has a mp 85°C i.e., much lower than the pure product (mp 172-175°C).
Characteristic Features It is obtained as monoclinic plates from alcohol and water. Its physical parameters are: mp 172-175°C; [α]24D -106° (c = 1 in absolute alcohol); UVmax 235, 241.5, 250 nm (ε 19500, 22000, 14300). It is practically insoluble in water, but freely soluble in ethanol, benzene, chloroform, ether, acetone, carbon disulphide and also in dilute NaOH solution.
Identification It readily forms the corresponding methyl ester as methyl abietate (C21H32O2), which is colourless to yellow thick liquid bp 360-365°C, d2020 1.040, and n20D 1.530.
Uses
1. It is used for manufacture of esters (ester gums), such as: methyl, vinyl and glyceryl esters for use in lacquers and varnishes.
2. It is also employed extensively in the manufacture of ‘metal resinates’ e.g., soaps, plastics and paper sizes.
3. It also assists in the growth of butyric and lactic acid bacteria.
------------------------------------------------
* Harris, Sanderson, Org. Syn. Coll. Vol. IV, 1 (1963); and Fieser and Fieser, The chemistry of Natural Products Related
to Phenanthrene (New York, 3rd. edn., 1949).
** A.W. Burgastahler, and L.W. Worden., J. Am. Chem. Soc., 83, 2587, (1961) E. Wenkert et al., ibid, 86, 2038, (1964)