2.7 Alkaloids Derived from Tyrosine
The pyridoxal phosphate (PLP)-dependent decarboxylation of L-Tyrosine yields the simple phenylethylamine tyramine, that subsequently undergoes di-N-methylation thereby producing hordenine. Hordenine is regarded as a germination inhibitory alkaloid obtained from barely viz., Hordeum vulgare (Graminae/Poaceae).
There are a number of alkaloids derived from tyrosine which may be classified as stated below:
(i) Phenylethylamine alkaloids,
(ii) Simple Tetrahydro iso-quinoline alkaloids,
(iii) Modified tetrahydro iso-quinoline alkaloids,
(iv) Phenylethylisoquinoline alkaloids, and
(v) Amaryllidaceae alkaloids.
The various groups of alkaloids mentioned above shall now be treated individually in the sections that follows:
---------------------------------------
* Barger, Blackie, J. Chem. Soc. 743 (1936)
2.7.1 Phenylethylamine Alkaloids
The important alkaloids belonging to this category are, namely: Ephedrine, Hordenine, Mescaline and Narceine, which shall be discussed as under:
A. Ephedrine
Biological Source It is obtained from the dried tender stems of the Chinese wonder drug Ma Huang which is being used in the Chinese systems of Medicine for more than five thousand years. It occurs in Ephedra vulgaris Hook. F. (E. gerardiana Wall); Ephedra sinica Stapf. (1-3%); Ephedra equisetina Bunge. (2%) belonging to the natural order Gentaceae; and several other Ephedra species. Besides, it is also found in the roots of Aconitum napellus L. (Ranunculaceae) (Aconite, Monkshood, Blue Rocket); and Ephedra nevadensis S. Wats. (Ephedraceae) (Mormon Tea, Nevada Jointfir).
Chemical Structure
α-[1-(Methylamino)-ethyl] benzene-methanol; (C10H15NO).
Isolation Both ephedrine and pseudoephedrine may be extracted from the plant source by general procedures described earlier under alkaloid extraction, through successive and dilute HCl extraction procedures.
However, the separation of ephedrine from pseudoephedrine may be accomplished by means of their corresponding oxalate salts; the ephedrine oxalate being comparatively less soluble in cold water than pseudoephedrine oxalate separates out first.
Note: Chloroform is not ragarded as an appropriate solvent for extraction of ephedrine as it forms its corresponding ephedrine hydrochloride salt after its dissolution in CHCl3and subsequent evaporation of solvent.
Fermentation Method Ephedrine may be prepared on a commercial scale economically by the process of fermentation using a mixture of molasses (a by-product of sugar industry containing 8-10% of cane sugar i.e., C6H12O6) and benzaldehyde. The resulting keto-alcohol i.e., benzylhydroxy methyl ketone is subsequently mixed with a solution of methylamine and treated with hydrogen gas to yield a racemic mixture of ephedrine as given below:
Characteristic Features The characteristic features of some racemic forms, optical isomers and their respective salts are enumerated below:
1. dl-Ephedrine (Synonyms: Racephedrine; Racemic Ephedrine): The crystals have mp 79°C; and are soluble in oils, chloroform, ether, water, and ethanol.
2. dl-Ephedrine Hydrochloride (Synonyms: Ephetonin; Racephedrine Hydrochloride) (C10H15NO.HCl): The crystals have mp 187–188°C; and pH 6.0. Its solubility profile are: 1 gdissolves 4 ml water, 40 ml of 95% ethanol at 20°C; and practically insoluble in ether.
3. dl-Ephedrine Sulphate (Synonym: Racephedrine Sulphate) (C10H15NO.H2SO4): The crystals have mp 247°C, and are soluble in ethanol and water. Its solution has a pH of 6.0.
4. l-Ephedrine [L-Erythro-2(methylamino)-1-phenylpropan-1-ol): It is obtained as waxy solid, crystals or granules, having a soapy feel and the substance gradually decomposes on exposure to light. It may contain water upto ½ mole (5.2%). However, the anhydrous product is hygroscopic in nature having mp 34°C. Interestingly, the absorption of water enhances mp to 40°C; and bp 255°C. The pH of aqueous solution (1 in 200) is 10.8. 1 g of it dissolves in 20 ml water, 0.2 ml ethanol; and freely soluble in ether, chloroform and oils.
5. l-Ephdrine Hydrochloride (Synonyms: Ephedral; Senedrine): It is obtained as orthorhombic needles having mp 216-220°C, which are affected by light. Its specific optical rotation [α]D25 -33 to –35.5° (C = 5). The pH of aqueous solution (1 in 200) is 5.9. 1 g dissolves in 3 ml water, 14 ml ethanol; and is found to be practically insoluble in chloroform and ether.
6. l-Ephedrine Sulphate: Its orthorhombic needles have mp 245°C (decomposed) and are affected by light. Its specific optical rotation [α]D25 -29.5 to –32.0° (C = 5). 1 g dissolves in 1.2 ml water and 95 ml ethanol; and freely soluble in hot alcohol. Its pH is about 6.
Identification Tests
1. Dissolve 0.01 g of ephedrine in 1 ml water by adding a few drops of dilute HCl. To this add two drops of CuSO4 solution (5% w/v) followed by a few-drops of NaOH solution when a reddish colour is developed. Now, add 2-3 ml ether and shake the contents thoroughly; the ethereal layer turns purple while the lower aqueous layer becomes blue.
2. Dissolve 0.2 g of ephedrine in 30 ml of chloroform in a stoppered flask and shake the contents vigorously. Allow the mixture to stand for at least 12 hours at room temperature and then remove the chloroform over an electric water bath. The crystals of ephedrine hydrochloride separate out.
3. Triturate 0.05 g of ephedrine with a few crystals of [K3Fe(CN)6] i.e., potassium ferricyanide, followed by a few drops of water and heat on a water bath slowly when a distinct odour of benzaldehyde (i.e., similar to the odour of bitter almonds) in given out.
Uses
l. l-Ehedrine is used extensively as a bronchodilator.
2. It also exerts excitatory action on the CNS and produces noticeable effects on skeletal muscles.
3. It is also employed as nasal decongestant.
B. Hordenine
Synonyms Anhaline; Eremursine; Peyocactine.
Biological Sources It is obtained from the plant of Lophophora williamsii (Lamaire) Coult. (Catctaceae) (Peyote) and Selenicereus grandiflorus Britt and Rose (Coctaceae) (Night Blooming Cereus).
Chemical Structure
4-[2-Dimethylamino) ethyl] phenol; (C10H15NO).
Isolation It is isolated from barley germs by the method suggested by Erspamer and Falconieri* (1952).
Characteristic Features
1. It is obtained as orthorhombic prisms from ethanol or benzene +ether; as needles from water having mp 117-118°C.
2. It sublimes at 140-150°C and has a bp11 173°C.
3. Solubility Profile: It is very soluble in chloroform, ethanol and ether; 7 g dissolves in 1 L of water; practically insoluble in petroleum ether; and sparingly soluble in benzene, xylene and toluene.
Identification Test Hrodenine readily forms its hydrochloride salt which is obtained as needles from ethanol having mp 177°C; and it is very soluble in water.
Uses It exhibits digitalis-like activity.
C. Mescaline
Synonym Mezcaline
Biological Sources It is obtained from Peyote (Mescal Buttons) the flowering heads of Lophophore williamsii (Lemaire) Coult. (Coctaceae) and the cactus Trichocereus pachanoi Britton and Rose (Cactaceae) (Achuma, San Pedro Aguacolli).
Chemical Structure
3, 4, 5-Trimethoxybenzeneethanamine; (C11H17NO3).
Isolation Mescaline has been successfully isolated from the plant source by Banholzer et al.* (1952).
Characteristic Features
1. The crystals have mp 35-36°C and bp12 180°C.
2. It is moderately soluble in water; freely soluble in ethanol, chloroform and benzene; and practically insoluble in ether and petroleum ether.
Identification Tests It forms readily a variety of salts, such as:
1. Mescaline Hydrochloride (C11H17NO3C11H17NO3.HCl): The needles have mp 181°C and freely soluble both in ethanol and water.
2. Mescaline Sulphate Dihydrate [(C11H17NO3)2.H2SO4.2H2O)]: It is obtained as prisms having mp 183-186°C; soluble freely in methanol and hot water; and sparingly soluble in ethanol and cold water.
3. Mescaline Acid Sulphate (C11H17NO3.H2SO4): The crystals have mp 158°C.
4. N-Acetylmescaline: It mostly occurs naturally, mp 94°C.
5. N-Methylmescaline: It occurs naturally, bp 130-140°C.
6. N-Benzoylmescaline: It is obtained as needles from aqueous ethanol having mp 121°C; and is found to be very soluble in ether and ethanol.
Note: This is a controlled substance (hallucinogen) listed in the US code of Federal Regulations
[Title 21 Part 1308.11 (1995)].
---------------------------------------------------
* Erspamer and Falconieri, Naturniss, 39, 431 (1952).
D. Narceine
Biological Source It is obtained from the dried latex (opium) by incision from the unripe capsule of Papaver somniferum Linn., (Papaveraceae) to the extent of 0.1-0.5%.
Chemical Structure
6-[[6-[2-(Dimethylamino) ethyl]-4-methoxy-1, 3-benzodioxol-5 yl] acetyl]-2, 3-dimethoxy benzoic acid; (C23H24NO8).
Isolation The isolation of nareceine from morphine mother liquors is tedious.** It may also be prepared from narcotine or gnoscopine.***
Characteristic Features
1. The anhydrous material is very hygroscopic in nature having mp 138°C; and it: uvmax (ethanol) is 270 nm (log € 3.98).
2. Usually the alkaloid is obtained as the trihydrate.
3. The clusters of silky and prismatic needles are obtained from water having mp 176°C.
4. Its dissociation constants are pKb at 20° = 10.7; Kb = 2 × 10–11; pka = 9.3; Ka = 5 × 10–10.
5. Th pH of its saturated solution is 5.8.
6. Solubility Profile: 1g dissolves in 770 ml water; 220 ml boiling water; moderately soluble in hot alcohol; almost insoluble in benzene, chloroform, ether, petroleum ether.
7. It forms salts with solutions of alkali hydroxide and also with dilute mineral acids.
Identification Test Ethylnarceine Hydrochloride (C25H32ClNO8) (Synonym: Narcyl): It is obtained as plates from water having mp 208-210°C. It is slightly soluble in cold water, insoluble in ether; and freely soluble in hot water, ethanol and chloroform.
Uses
1. Narcyl is used as a narcotic analgesic.
2. Narcyl is also employed as an antitussive agent.
Biosynthesis of Hordenine and Mescaline Decarboxylation of L-tyrosine via pyridoxal phosphate (PLP) yields the simple phenylethylamine derivative tyramine, which an di-N-methylation gives rise to hordenine. Besides, phenylethylamine derivatives commonly exhibit either 3, 4-di- or 3, 4, 5-trihydroxylation reactions, and are subsequently derived via dopamine i.e., the decarboxylation product obtained from L-DOPA (L-dihydroxyphenylalaline). The two variants of catecholamines,namely: first, a mammalian neurotransmitter noradrenaline (norepinephrine), and secondly, the most common ‘fight or flight’ hormone released in animals from the adrenal gland due to fear phychosis or stress adrenaline (epinephrine). Furthermore, these two compounds are formed due to β-hydroxylaton and N-methylation of dopamine.
Lastly, aromatic hydroxylation and O-methylation convert dopamine into mescaline. All these reactions have been shown sequentially as given below.
-------------------------------------------
* Banholzer et al. Helv. Chim. Acta, 35, 1577 (1952).
** Merek, Chem. Ztg., 13, 525 (1889)
*** Roser, Ann. 247, 167 (1888).
2.7.2 Simple Tetrahydro Isoquinoline Alkaloids
The typical representatives of the simple tetrahydroisoquinoline derivatives are the closely-related alkaloids occurring along with mescaline are, namely: anhalamine, anhalonine and anhalonidine.
These three alkaloids shall be discussed in the pages that follow:
A. Anhalamine
Biological Sources It is obtained from the plant Lophophora williamsii (Lemaire) Coult. (Coctaceae) (Peyote), and Anhalonium lewinii. Henn. (Cactaceae).
Chemical structures
1, 2, 3, 4-Tetrahydro-6, 7-dimethoxy-8-isoquinolinol, (C11H15NO3).
Characteristic Features
1. The crystals have mp 189-191°C.
2. Its uvmax (ethanol) is 274 nm (log € 2.90).
3. Solubility Profile: It is found to be almost insoluble in cold water, cold ethanol, ether and freely soluble in hot water, ethanol, acetone and dilute acids.
Identification Test Anhalamine Hydrochloride Dihydrat (C11H15NO3.HCl.2H2O): It is obtained as crystals from water having mp 258°C.
Uses It may play a minor role in causing hallucinatious.
B. Anhalonine
Synonym Anhalanine
Biological Sources It is obtained from the mescal buttons [Lophophora williamsii (Lemaire) Coult. (Anhalonium lewinii Henn). Cactaceae]; and also in Ariocarpus, in Gymnocalycium gibbosum.
Chemical Structure
6, 7, 8, 9-Tetrahydro-4-methoxy-9-methyl-1, 3-dioxolo [4, 5-h] isoquinoline, (C12H15NO3).
Characteristic Features
1. It is obtained as rhombic needles from petroleum ether having mp 86°C and bp0.02 140°C.
2. Its specific optical rotation [α]D25-63.80 (methanol); and [α]D25 -56.30 (chloroform).
3. It is found to be freely soluble in ethanol, ether, chloroform, benzene and petroleum ether.
Identification Test
Anhalonine Hydrochloride (C12H15NO3.HCl): It is obtained as orthorhombic prisms decomposing at 255°C. Its aqueous solution is almost neutral. It is found to be freely soluble in hot water.
Uses It may be employed as a mild hallucinating agent.
C. Anhalonidine
Biological Source It is invariably obtained from the mescal buttons, the buds of
Lophophora williamsii (Lemaire) Coult. (Anhalonium lewinii Henn.) belonging to the natural order Coctaceae.
Chemical Structure
1, 2, 3, 4-Tetrahydro-6, 7-dimethoxy-1-methyl-8-isoquinolinol; (C12H17NO3).
Characteristic Features
1. It is mostly obtained as small octahedral crystals from benzene having mp 160-161°C.
2. Its uvmax(ethanol) is 270 nm (log € 2.81).
3. Its aqueous solution acts as a strong base.
4. It is freely soluble in water, ethanol, chloroform and hot benzene; sparingly soluble in ether; and practically insoluble in petroleum ether.
5. It has been observed that the solutions of anhalonidine acquire a reddish colouration on standing.
Uses It may be used as a mild hallucinogen.
Biosynthesis of Anhalamine, Anhalonine and Anhalonidine Interestingly, the two additional C-atoms present in anhalonidine and anhalonine are provided by pyruvate; whereas, the C-atom for anhalamine is supplied by glyoxylate, as shown below. However, in each instance, a carboxyl group is lost from this aforesaid additional precursor. The pyruvate i.e., the keto-acid eventually reacts with an appropriate phenylethylamine, in this particular instance the dimethoxy-hydroxyderivative, thereby yielding a Schiff Base. Further, a Mannich-like mechanism helps in the cyclization to produce the heterocyclic isoquinoline nucleus, whereby the mesomeric effect of an oxygen substituent caters for the nucleophilic site on the aromatic ring. Evidently, restoration of aromaticity via proton loss yields the tetrahydroquinoline nucleus, thus representing overall a biosynthetic equivalent of the Pictet-Spengler Isoquinoline Synthesis.* Subsequently, the carboxyl group is eliminated, not by means of a simple decarboxylation process, but via an unusual oxidative decarboxylation process that essentially involves the following steps, namely:
(i) First, producing the intermediate imine,
(ii) Secondly, subjecting to reduction yielding anhalonidine,
(iii) Thirdly, subjecting to methylation giving rise to anhalonine,
(iv) Fourthly, subjecting phenylethylamine precursor employing the glyoxylic acid instead of pyruvic acid generating anhalamine.
2.7.3 Modified Benzyltetrahydroisoquinoline Alkaloids
The modification of benzyltetrahydroisoquinoline nucleus to certain other types of alkaloid(s) could be accomplished by virtue of phenolic oxidative coupling.
Interestingly, the coupling of two benzyltetrahydroisoquinoline molecules via ether bridges result into the formation of two important alkaloids, namely: tetrandrine and tubocurarine, as given below.
It is, however, pertinent to mention here that the aforesaid mode of coupling is perhaps less frequently found than that involving carbon-carbon bonding between aromatic rings. The major opium alkaloids viz., morphine, codeine and thebaine are obtained through this mode of coupling. (R)-Reticuline has been established beyond any reasonable doubt as the precursor of the above threemorphinan alkaloids. Interestingly, there exist an ample evidence to show that the later stages of the proposed biosynthetic pathway undergo modifications in certain strains of opium poppy. Thus, in such modified strains of opium poppy thebaine is being converted to oripavine and morphinone,whereby the phenolic O-methyl moiety is removed before that of the ether, i.e., the same steps are carried out but in an altogether different order.
The various alkaloids belonging to this category, namely: morphine, codeine, thebaine, reticuline, oripavine and morphinone shall be discussed separately in the following sections:
A. Morphine
Synonyms Morphium; Morphia; Dolcontin; Duromorph; Morphina; Nepenthe.
Biological Sources Morphine is obtained from a variety of medicinal plants, such as: Argemone mexicana L. (Papaveraceae) (Prickly Poppy); Eschscholzia californica Cham. (Papaveraceae) (California Poppy); Papaver bracteatum Lindl. (Papaveraceae) (Great Scarlet Poppy; Thebaine Poppy); Papaver somniferum L. (Papaveraceae) (Opium Poppy; and Poppyseed Poppy Keshi).
Chemical Structure
(5α, 6α)-7, 8-Didehydro-4, 5-epoxy-17-methylmorphinan-3, 6-diol; (C17H19NO3).
Isolation The latex obtained by incision on the unripe capsule of opium poppy is first collected in clean, plastic containers, and the process of incision is repeated at least four times on the same capsule after an interval of two days. Care must be taken to make the incisions on the superficial surface only so as to collect exclusively the external exudation of latex. Subsequently, the latex is dried carefully either by exposing to air on metallic shallow plates or by passing a stream of hot air.
Thus the ‘opium’ or the dried latex is stored for the isolation of morphine. It is found to contain usually 9.5% morphine when calculated as anhydrous morphine.
The morphine may be isolated form ‘Powdered Opium’ by adopting the following steps sequentially:
Step-1: The powdered opium is shaken with calcium chloride solution and filtered.
Step-2: The resulting filtrate is concentrated and to it is added 10% w/v sodium hydroxide solution carefully i.e., to solubilize morphine, codeine and narceine. It is now filtered.
Step-3: The filtrate containing morphine, codeine and narceine is extracted with chloroform. The resulting mixture is separated.
Step-4: The lower chloroform layer contains codeine, whereas the upper aqueous layer comprises of morphine and narceine.
Step-5: The aqueous layer is first acidified and subsequently made alkaline with ammonia, whereby morphine gets precipitated and collected as a while solid residue (Yield = 9.5%).
Characteristic Features
1. Morphine is obtained as short, orthorhombic, columnar prisms from anisole that gets decomposed at 254°C. It also occurs in its metastable phase having mp 197°C. However, the high melting form sublimes at 190-200°C (0.2 mm pressure at 2 mm distance).
2. It has a bitter taste.
3. Morphine (free-base) unlike most other alkaloids in their free-base forms is found to be sparingly soluble in chloroform and nearly insoluble in ether or benzene.
4. Morphine gets dissolved in caustic alkalies by virtue of the fact that the OH moiety at C-3 is phenolic in nature and the other OH function at C-6 is a secondary alcoholic group.
5. Morphine is a monoacidic base and hence, forms salts that crystallizes rapidly. These are found to be neutral to litmus and methyl orange.
6. The average pH of a saturated solution of morphine salt is found to be 4.68.
Note: Morphine reduces iodic acid and potassium iodate.
4. Sodium Nitrite Test: To a solution of morphine in dilute HCl add a few drops of sodium nitrite solution (1% w/v). Allow the reaction mixture to stand for 5-8 minutes and then make it alkaline with dilute ammonia solution, the development of a red colour confirms the presence of morphine.
Note: (1) It is a non-specific test for morphine and is also given by other phenolic substances.
(2) It legitimately distinguishes morphine from codeine.
5. Nitric Acid Test: Morphine readily gives an orange-red colouration when a few mg of it is treated with a few drops of concentrated nitric acid.
(a) The resulting orange-red colouration rapidly changes to yellow on heating.
(b) The orange-red colouration gets easily disappeared on the addition of a few drops of stannous chloride solution (SnCl2) (1% w/v).
6. Ferric Chloride Test: When a neutral solution of morphine is treated with a few drops of ferric-chloride solution (1% w/v), a greenish-blue colour is produced.
Derivatives of Morphine A number of derivatives of morphine are produced that essentially have distinct characteristic features as enumerated below:
1. Morphine Monohydrate (C17H19NO3.H2O):
(i) It is obtained as orthorhombic, sphenoidal prisms, or needles from methanol that gets decomposed at 254-256°C with rapid heating.
(ii) It darkens on exposure to light and also loses water of crystallization at 130°C.
(iii) Its physical parameters are: dD20 1.32; [α]D25 -1320(methanol); pKb at 20°C = 6.13, pKa 9.85; pH of a saturated solution 8.5; and uvmax in acid: 2.85 nm, in alkali: 298 nm.
(iv) Solubility Profile: 1 g dissolves in about 5000 ml of water, 1100 of boiling water, 210 ml of ethanol, 98 ml of boiling ethanol, 1220 ml of chloroform, 6250 ml of ether, 114 ml of amyl alcohol, 10 ml of boiling methanol, 525 ml of ethyl acetate; freely soluble in solutions of fixed alkali and other alkaline earth hydroxides, in phenols, cresols; moderately soluble in mixtures of chloroform with alcohols; and slightly soluble in ammonia benzene.
2. Morphine Acetate Trihydrate (C19H23NO5.3H2O):
(i) It is a yellowish-white powder.
(ii) It has a slight acetic odour.
(iii) It specific optical rotation [α]D15 -770 (water).
(iv) It dissolves 1 g in 2.25 ml of water, 2 ml of boiling water, 22 ml of ethanol, 2 ml of ethanol at 60°C, 4.5 ml of glycerol, 4.75 ml of chloroform; and practically insoluble in ether.
3. Morphine Tartrate Tihydrate [(C17H19NO3)2.C4H6O6.3H2O)]: It is obtained as a crystalline powder. It is soluble in 11 parts of water; slightly soluble in alcohol; and practically insoluble in ether, chloroform and carbon disulphide.
Uses
1. It is used as a potent narcotic analgesic.
2. It is usually given in severe pains and also in such instances where patient fails to show positive response to other analgesics.
3. It exerts a biphasic action on the CNS.
4. It is found to sedate the respiratory centre, emetic centre and the cough centre through its action in the medulla.
5. It stimulates the chemoreceptor-trigger-zone located in the medulla that ultimately causes nausea and vomilting; and this is perhaps regarded as a side-effect.
6. It also exerts sedative and hypnotic actions.
Note: Morphine and its salts are habit forming drugs. Hence, its use must be done under the strict observation of a physician.
B. Codeine
Synonyms Codicept; Morphine monomethyl ether; Morphine 3-methyl ether; Methylmorphine.
Biological Sources It is obtained from the plant Argemone mexicana L. (Papaveraceae) (Prickly Poppy); Eschscholzia california Cham. (Papaveraceae) (California Poppy); Papaver bracteatum Lindl. (Papaveraceae) (Great scarlet poppy, Thebaine Poppy); and Papaver somniferum L. (Papaveraceae) (Opium Poppy, Poppyseed Poppy Keshi).
Chemical Structure
(5α, 6α)-7, 8-Didehydro-4, 5-epoxy-3-methoxy-17-methyl-morphinan-6-ol; (C18H21NO3).
Preparation It is invariably present in opium from 0.7 to 2.5% depending on the sources of plant substances. However, mostly it is prepared by carrying out the methylation of morphine.
Characteristic Features
1. It is obtained as monohydrate orthorhombic sphenoidal rods or tablets (octahedra) from water or dilute ethanol having mp 154-156°C (after drying at 80°C).
2. It is found to sublime (when anhydrous) at 140-145°C under 1.5 mm reduced pressure.
3. It is observed to melt to oily drops when heated in an amount of water is sufficient for complete solution, and subsequently crystallizes on cooling.
4. Its physical parameters are: d420 1.32; [α]D15 -1360(C = 2 in ethanol); [α]D15 – 112o (C = 2 in chloroform); pK (15°) 6.05; pH of a saturated solution 9.8.
5. Solubility Profile: 1 g dissolves in 120 ml water, 60 ml water at 80°C, 2 ml ethanol, 1.2 ml hot ethanol, 13 ml benzene, 18 ml ether, 0.5 ml chloroform; freely soluble in methanol, dilute acids and amyl alcohol; and almost insoluble in solutions of alkali hydroxides and in petroleum ether.
Identification Test It forms various types of salts, namely:
1. Codeine Acetate (C20H25NO5): The dihydrate is obtained as crystals having an acetic acid odour. It is found to be soluble in water and ethanol. It loses acetic acid on keeping and subsequently turns into a product which is incompletely soluble in water.
2. Codeine Hydrobromide (C18H21NO3.HBr): The dihydrate is obtained as crystals and the anhydrous product shows a mp 190-192°C; [α ]D22 – 96.60 ; 1 g dissolves in 60 ml water, 110 ml ethanol; and pH about 5.
3. Codeine Hydrochloride (C18H21NO3.HCl): Its dihydrate salt is obtained as small needles having mp ~ 280°C with some decomposition; [α ]D22 -1080 ; 1 g dissolves in 20 ml of water, 1 ml boiling water, 180 ml ethanol; and pH about 5.
4. Codeine Salicylate (C25H27NO6): It is obtained as white crystalline powder; slightly soluble in water; and freely soluble in ethanol or ether.
5. Codeine Phosphate (C18H24NO7P) (Galcodine): The hemihydrate salt (USP) is obtained as fine, white, needle-shaped crystals or crystalline powder. It is odorless and affected by light. The solution is acidic to litmus. It is freely soluble in water; very soluble in hot water; slightly soluble in ethanol; and more soluble in boiling ethanol.
6. Codeine Sulphate (C36H44N2O10S): The trihydrate is obtained as crystals or crystalline powder; 1 g dissolves in 30 ml water; 6.5 ml water at 80°C; 1300 ml ethanol; insoluble in chloroform or ether; pH 5.0.
7. Codeine Methyl Bromide (C19H24Br-NO3) (Eucodin) : Its crystals have mp ~ 260°C; soluble in 2-3 parts of water, in hot methanol; sparingly soluble in ethanol; and insoluble in chloroform and ether.
Uses
1. It is mostly used as a narcotic analgesic.
2. It is invariably employed as an antitussive.
C. Thebaine
Synonym Paramorphine;
Biological Sources It is obtained from the fresh capsule latex (0.125%), dried 0.25 to 0.26% of Papaver bracteatum Lindl. (Papaveraceae) (Great Scarlet Poppy, Thebaine Poppy); and the airdried milky exudation obtained from excised unripe fruits of Papaver somniferium L. (Papaveraceae)
(Opium Poppy, Poppyseed Poppy Keshi).
Chemical Structure
(5α)-6, 7, 8, 14-Tetrahydro-4, 5-epoxy-3, 6-dimethoxy-17-methylmorphinan; (C19H21NO3).
Isolation Thebaine may be isolated from opium by means of the following steps, namely:
Step-1: Opium (dried latex) is treated with calcium chloride solution and then extracted with warm water. Allow it to remain as such for 24 hours.
Step-2: Filter the resulting product and collect the residue and filtrate separately.
Residue—contains the salts of calcium as lactate, sulphate, resinate and meconate (To be discarded).
Filtrate— contains the hydrochloride of various alkaloids present in opium.
Step-3: Add dilute NaOH solution (2N) carefully to the resulting filtrate and allow it to stand for 4- 6 hours. Filter the contents of the flask:
Filtrate—contains morphine, codeine and narceine
Residue—contains thebaine, papaverine and narcotine
Step-4: Dissolve the residue or precipilate in dilute ethanol (50% v/v), make slightly acidic with the addition of dilute glacial acetic acid and finally add to it approximately three volumes of boiling distilled water.
Step-5: Filter the above reaction product:
Filtrate—contains thebaine
Residue—contains papaverine and narcotine
Step-6: Concentrate the filtrate obtained in Step-5 under reduced pressure and add to it dilute NH4OH solution to make it alkaline; and extract the liberated alkaloid thebaine successively with chloroform. Thebaine is obtained after evaportion of chloroform under vaccuo.
Characteristic Features
1. It is obtained as orthorhombic, rectangular plates by sublimation at 170-180°C under atmospheric pressure and a 1 mm distance mp 193°C (rapid heating).
2. Its physical parameters are: [α]D15 -2190(p = 2 in ethanol); [α]D23(p = 5 in chloroform); pK at 15°C = 6.05; and pH of a saturated solution is 7.6.
3. Solubility Profile: 1 g dissolves in 1460 ml water at 15°C, in about 15 ml hot ethanol, 13 ml chloroform, 200 ml ether, 25 ml benzene, 12 ml pyridine; and not very soluble in petroleum ether.
Identification Tests Thebaine forms a number of salt derivatives which have specific characteristic features, such as:
1. Thebaine Salicylate (C19H21NO3.C7H6O3): It is obtained as crystals which are soluble in 750 parts of water. Thus, thebaine may be separated from other major alkaloids of opium by forming its salicylate derivative which is sparingly soluble in water.
2. Thebaine Hydrochloride Monohydrate (C19H21NO3.HCl.H2O): It is obtained as orthorhombic prisms from alcohol having [α]D23 -1640(p = 2). It is found to be soluble in about 12 parts of water and in ethanol. The pH of a 0.05 molar solution is 4.95.
3. Thebaine Oxalate Hexahydrate (2 C19H21NO3.C2H2O4.6 H2O): It is obtained as prisms. It is soluble in 10 parts of water and also in ethanol; and is almost insoluble in ether.
4. Thebaine Binoxalate Monohydrate (C19H21NO3.C2H2O4.H2O): It is obtained as prisms and found to be soluble in 45 parts of water.
5. Thebaine Bitartrate Monohydrate (C19H21NO3.C4H6O6.H2O): It is obtained as prisms, soluble in 130 parts of water, quite soluble in both hot water and hot ethanol.
6. It gives a red colour on the addition of a few drops of cold sulphuric acid which ultimately changes to orange yellow.
Uses It is an opiate analgesic.
D. Reticuline
Synonym Coclanoline.
Biological Sources It is obtained from the plant Hydratis canadensis L. (Ranunculaceae) (Goldenseal); the leaves of Laurus nobilis L. (Lauraceae) (Bay, Grecian Laurel, Green Bay); the air-dried milky exudation obtained from excised unripe fruits of Papaver somiferum L. (Papaveraceae) (Opium Poppy, Poppyseed Poppy Keshi); and the leaves of Sassafras albidum (Nutt.) Nees (Lauraceae) (Sassafras).
Chemical Structure
1, 2, 3, 4-Tetrahydro-1-[(3-hydroxy-4-methoxyphenyl) methyl]-6-methoxy-2-methyl-7-isoquinolinol; (C19H23NO4).
Isolation Gopinath et al.,* has described the isolation of d-form of reticuline from Anona reticulata Linn., (Annonaceae).
Characteristic Features
1. The dl-form of reticuline is obtained as pink crystals having mp 146°C.
2. The uvmax: 284 nm (log € 3.85).
3. Solubility Profile: It is soluble in aqueous buffer of pH < 7.5 or > 11; and is practically insoluble in water at pH 8-10.
Identification Tests
(S)-Form Reticuline Perchlorate (C19H23NO4.HClO4): It is obtained as colourless prisms from ethanol having mp 203-204°C. Its specific optical rotation [α]D18 +88.3o (C = 0.21 in ethanol).
-------------------------------------
* Gopinath et al., Ber. 92, 776 (1959).
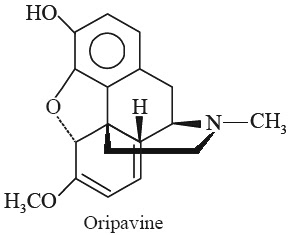
E. Oripavine
Synonym O3-Demethylthebaine.
Biological Sources It is obtained from the plant Papaver bracteatum Lindl. (Papaveraceae) (Great Scarlet Poppy, Thebaine Poppy); and Papaver orientale Linn. (Papaveraceae).
Chemical Structure
(5α)-6, 7, 8, 14-Tetrahydro-4, 5-epoxy-6-methoxy-17-methyl-morphinan-3-ol; (C18H19NO3).
Isolation It has been isolated from plant source by Kiselev and Konovalova.*
Characteristic Features The crystals have mp 200-201°C; and [α]D20 -211.80.
Identification Tests
1. Oripavine Hydrochloride (C18H19NO3.HCl): It is obtained as crystals which decompose at 244-245°C.
2. Oripavine Methiodide (C18H19NO3.CH3I): The crystals decompose at 207-208°C.
F. Morphinone It has been observed that the later stages of the biosynthetic pathway starting from reticuline leading to thebaine and morphine are strategically modified in some strains of opium poppy. Therefore, in such strains, thebaine is converted by way of oripavine and morphinone. In this pathway the phenolic O-methyl function is removed before that of the enol ether, i.e., accomplishing the same steps but in a different order. In other words, morphinone is obtained by the demethylation of oripavine as shown below:
Biosynthesis of Morphine, Codeine, Thebaine, Oripavine and Morphinone The various steps involved are as follows:
1. (R)-Reticuline, may be redrawn as shown in page 495 following pathway is found to be the substrate for one-electron oxidation via the phenol moiety present in each ring thereby yielding the diradical.
2. Subsequent coupling ortho to the phenol group in the tetrahydroisoquinoline nucleus, and para to the phenol in the benzyl substituent, gives rise to salutaridine—a dienone which is found as minor alkaloidal component in the opium poppy Papaver somniferum.
3. Thebaine is achieved via salutaridinol produced from salutaridine by means of the stereospecific reduction of the carbonyl group.
4. In thebaine the ring closure to form the ether linkage is caused due to the nucleophilic attack of the phenol moiety on the dienol system followed by a displacement of the hydroxyl group.
5. Future reactions essentially involve conversion of thebaine into morphine via codeine by virtue of a process that exclusively modifies the oxidation state of the diene ring, but apparently removes two O-methyl groups.
6. One is evidently present as an enol ether, removal of which yields neopinone, that subsequently gives rise to codeinone and then sodeine by the help of allylic isomerisation and reduction respectively.
7. In certain specific strains of opium poppy, thebaine is changed to oripavine and morphinone by virtue of the pathway that essentially removes the phenolic O-methyl function before that of the enol ether.
--------------------------------------------------------
* Kiselev and Konovalova J. Gen. Chem. USSR, 18, 142 (1948).