2.7.3 Modified Benzyltetrahydroisoquinoline Alkaloids
The modification of benzyltetrahydroisoquinoline nucleus to certain other types of alkaloid(s) could be accomplished by virtue of phenolic oxidative coupling.
Interestingly, the coupling of two benzyltetrahydroisoquinoline molecules via ether bridges result into the formation of two important alkaloids, namely: tetrandrine and tubocurarine, as given below.
It is, however, pertinent to mention here that the aforesaid mode of coupling is perhaps less frequently found than that involving carbon-carbon bonding between aromatic rings. The major opium alkaloids viz., morphine, codeine and thebaine are obtained through this mode of coupling. (R)-Reticuline has been established beyond any reasonable doubt as the precursor of the above three morphinan alkaloids. Interestingly, there exist an ample evidence to show that the later stages of the proposed biosynthetic pathway undergo modifications in certain strains of opium poppy. Thus, in such modified strains of opium poppy thebaine is being converted to oripavine and morphinone, whereby the phenolic O-methyl moiety is removed before that of the ether, i.e., the same steps are carried out but in an altogether different order.
The various alkaloids belonging to this category, namely: morphine, codeine, thebaine, reticuline, oripavine and morphinone shall be discussed separately in the following sections:
A. Morphine
Synonyms Morphium; Morphia; Dolcontin; Duromorph; Morphina; Nepenthe.
Biological Sources Morphine is obtained from a variety of medicinal plants, such as: Argemone mexicana L. (Papaveraceae) (Prickly Poppy); Eschscholzia californica Cham. (Papaveraceae) (California Poppy); Papaver bracteatum Lindl. (Papaveraceae) (Great Scarlet Poppy; Thebaine Poppy); Papaver somniferum L. (Papaveraceae) (Opium Poppy; and Poppyseed Poppy Keshi).
Chemical Structure
(5α, 6α)-7, 8-Didehydro-4, 5-epoxy-17-methylmorphinan-3, 6-diol; (C17H19NO3).
Isolation The latex obtained by incision on the unripe capsule of opium poppy is first collected in clean, plastic containers, and the process of incision is repeated at least four times on the same capsule after an interval of two days. Care must be taken to make the incisions on the superficial surface only so as to collect exclusively the external exudation of latex. Subsequently, the latex is dried carefully either by exposing to air on metallic shallow plates or by passing a stream of hot air.
Thus the ‘opium’ or the dried latex is stored for the isolation of morphine. It is found to contain usually 9.5% morphine when calculated as anhydrous morphine.
The morphine may be isolated form ‘Powdered Opium’ by adopting the following steps sequentially:
Step-1: The powdered opium is shaken with calcium chloride solution and filtered.
Step-2: The resulting filtrate is concentrated and to it is added 10% w/v sodium hydroxide solution carefully i.e., to solubilize morphine, codeine and narceine. It is now filtered.
Step-3: The filtrate containing morphine, codeine and narceine is extracted with chloroform. The resulting mixture is separated.
Step-4: The lower chloroform layer contains codeine, whereas the upper aqueous layer comprises of morphine and narceine.
Step-5: The aqueous layer is first acidified and subsequently made alkaline with ammonia, whereby morphine gets precipitated and collected as a while solid residue (Yield = 9.5%).
Characteristic Features
1. Morphine is obtained as short, orthorhombic, columnar prisms from anisole that gets decomposed at 254°C. It also occurs in its metastable phase having mp 197°C. However, the high melting form sublimes at 190-200°C (0.2 mm pressure at 2 mm distance).
2. It has a bitter taste.
3. Morphine (free-base) unlike most other alkaloids in their free-base forms is found to be sparingly soluble in chloroform and nearly insoluble in ether or benzene.
4. Morphine gets dissolved in caustic alkalies by virtue of the fact that the OH moiety at C-3 is phenolic in nature and the other OH function at C-6 is a secondary alcoholic group.
5. Morphine is a monoacidic base and hence, forms salts that crystallizes rapidly. These are found to be neutral to litmus and methyl orange.
6. The average pH of a saturated solution of morphine salt is found to be 4.68.
Note: Morphine reduces iodic acid and potassium iodate.
4. Sodium Nitrite Test: To a solution of morphine in dilute HCl add a few drops of sodium nitrite solution (1% w/v). Allow the reaction mixture to stand for 5-8 minutes and then make it alkaline with dilute ammonia solution, the development of a red colour confirms the presence of morphine.
Note: (1) It is a non-specific test for morphine and is also given by other phenolic substances.
(2) It legitimately distinguishes morphine from codeine.
5. Nitric Acid Test: Morphine readily gives an orange-red colouration when a few mg of it is treated with a few drops of concentrated nitric acid.
(a) The resulting orange-red colouration rapidly changes to yellow on heating.
(b) The orange-red colouration gets easily disappeared on the addition of a few drops of stannous chloride solution (SnCl2) (1% w/v).
6. Ferric Chloride Test: When a neutral solution of morphine is treated with a few drops of ferric-chloride solution (1% w/v), a greenish-blue colour is produced.
Derivatives of Morphine A number of derivatives of morphine are produced that essentially have distinct characteristic features as enumerated below:
1. Morphine Monohydrate (C17H19NO3.H2O):
(i) It is obtained as orthorhombic, sphenoidal prisms, or needles from methanol that gets decomposed at 254-256°C with rapid heating.
(ii) It darkens on exposure to light and also loses water of crystallization at 130°C.
(iii) Its physical parameters are: dD20 1.32; [α]D25 -1320(methanol); pKb at 20°C = 6.13, pKa 9.85; pH of a saturated solution 8.5; and uvmax in acid: 2.85 nm, in alkali: 298 nm.
(iv) Solubility Profile: 1 g dissolves in about 5000 ml of water, 1100 of boiling water, 210 ml of ethanol, 98 ml of boiling ethanol, 1220 ml of chloroform, 6250 ml of ether, 114 ml of amyl alcohol, 10 ml of boiling methanol, 525 ml of ethyl acetate; freely soluble in solutions of fixed alkali and other alkaline earth hydroxides, in phenols, cresols; moderately soluble in mixtures of chloroform with alcohols; and slightly soluble in ammonia benzene.
2. Morphine Acetate Trihydrate (C19H23NO5.3H2O):
(i) It is a yellowish-white powder.
(ii) It has a slight acetic odour.
(iii) It specific optical rotation [α]D15 -770 (water).
(iv) It dissolves 1 g in 2.25 ml of water, 2 ml of boiling water, 22 ml of ethanol, 2 ml of ethanol at 60°C, 4.5 ml of glycerol, 4.75 ml of chloroform; and practically insoluble in ether.
3. Morphine Tartrate Tihydrate [(C17H19NO3)2.C4H6O6.3H2O)]: It is obtained as a crystalline powder. It is soluble in 11 parts of water; slightly soluble in alcohol; and practically insoluble in ether, chloroform and carbon disulphide.
Uses
1. It is used as a potent narcotic analgesic.
2. It is usually given in severe pains and also in such instances where patient fails to show positive response to other analgesics.
3. It exerts a biphasic action on the CNS.
4. It is found to sedate the respiratory centre, emetic centre and the cough centre through its action in the medulla.
5. It stimulates the chemoreceptor-trigger-zone located in the medulla that ultimately causes nausea and vomilting; and this is perhaps regarded as a side-effect.
6. It also exerts sedative and hypnotic actions.
Note: Morphine and its salts are habit forming drugs. Hence, its use must be done under the strict observation of a physician.
B. Codeine
Synonyms Codicept; Morphine monomethyl ether; Morphine 3-methyl ether; Methylmorphine.
Biological Sources It is obtained from the plant Argemone mexicana L. (Papaveraceae) (Prickly Poppy); Eschscholzia california Cham. (Papaveraceae) (California Poppy); Papaver bracteatum Lindl. (Papaveraceae) (Great scarlet poppy, Thebaine Poppy); and Papaver somniferum L. (Papaveraceae) (Opium Poppy, Poppyseed Poppy Keshi).
Chemical Structure
(5α, 6α)-7, 8-Didehydro-4, 5-epoxy-3-methoxy-17-methyl-morphinan-6-ol; (C18H21NO3).
Preparation It is invariably present in opium from 0.7 to 2.5% depending on the sources of plant substances. However, mostly it is prepared by carrying out the methylation of morphine.
Characteristic Features
1. It is obtained as monohydrate orthorhombic sphenoidal rods or tablets (octahedra) from water or dilute ethanol having mp 154-156°C (after drying at 80°C).
2. It is found to sublime (when anhydrous) at 140-145°C under 1.5 mm reduced pressure.
3. It is observed to melt to oily drops when heated in an amount of water is sufficient for complete solution, and subsequently crystallizes on cooling.
4. Its physical parameters are: d420 1.32; [α]D15 -1360 (C = 2 in ethanol); [α]D15 – 112o (C = 2 in chloroform); pK (15°) 6.05; pH of a saturated solution 9.8.
5. Solubility Profile: 1 g dissolves in 120 ml water, 60 ml water at 80°C, 2 ml ethanol, 1.2 ml hot ethanol, 13 ml benzene, 18 ml ether, 0.5 ml chloroform; freely soluble in methanol, dilute acids and amyl alcohol; and almost insoluble in solutions of alkali hydroxides and in petroleum ether.
Identification Test It forms various types of salts, namely:
1. Codeine Acetate (C20H25NO5): The dihydrate is obtained as crystals having an acetic acid odour. It is found to be soluble in water and ethanol. It loses acetic acid on keeping and subsequently turns into a product which is incompletely soluble in water.
2. Codeine Hydrobromide (C18H21NO3.HBr): The dihydrate is obtained as crystals and the anhydrous product shows a mp 190-192°C; [α ]D22 – 96.60 ; 1 g dissolves in 60 ml water, 110 ml ethanol; and pH about 5.
3. Codeine Hydrochloride (C18H21NO3.HCl): Its dihydrate salt is obtained as small needles having mp ~ 280°C with some decomposition; [α ]D22 -1080 ; 1 g dissolves in 20 ml of water, 1 ml boiling water, 180 ml ethanol; and pH about 5.
4. Codeine Salicylate (C25H27NO6): It is obtained as white crystalline powder; slightly soluble in water; and freely soluble in ethanol or ether.
5. Codeine Phosphate (C18H24NO7P) (Galcodine): The hemihydrate salt (USP) is obtained as fine, white, needle-shaped crystals or crystalline powder. It is odorless and affected by light. The solution is acidic to litmus. It is freely soluble in water; very soluble in hot water; slightly soluble in ethanol; and more soluble in boiling ethanol.
6. Codeine Sulphate (C36H44N2O10S): The trihydrate is obtained as crystals or crystalline powder; 1 g dissolves in 30 ml water; 6.5 ml water at 80°C; 1300 ml ethanol; insoluble in chloroform or ether; pH 5.0.
7. Codeine Methyl Bromide (C19H24Br-NO3) (Eucodin) : Its crystals have mp ~ 260°C; soluble in 2-3 parts of water, in hot methanol; sparingly soluble in ethanol; and insoluble in chloroform and ether.
Uses
1. It is mostly used as a narcotic analgesic.
2. It is invariably employed as an antitussive.
C. Thebaine
Synonym Paramorphine;
Biological Sources It is obtained from the fresh capsule latex (0.125%), dried 0.25 to 0.26% of Papaver bracteatum Lindl. (Papaveraceae) (Great Scarlet Poppy, Thebaine Poppy); and the airdried milky exudation obtained from excised unripe fruits of Papaver somniferium L. (Papaveraceae)
(Opium Poppy, Poppyseed Poppy Keshi).
Chemical Structure
(5α)-6, 7, 8, 14-Tetrahydro-4, 5-epoxy-3, 6-dimethoxy-17-methylmorphinan; (C19H21NO3).
Isolation Thebaine may be isolated from opium by means of the following steps, namely:
Step-1: Opium (dried latex) is treated with calcium chloride solution and then extracted with warm water. Allow it to remain as such for 24 hours.
Step-2: Filter the resulting product and collect the residue and filtrate separately.
Residue—contains the salts of calcium as lactate, sulphate, resinate and meconate (To be discarded).
Filtrate— contains the hydrochloride of various alkaloids present in opium.
Step-3: Add dilute NaOH solution (2N) carefully to the resulting filtrate and allow it to stand for 4- 6 hours. Filter the contents of the flask:
Filtrate—contains morphine, codeine and narceine
Residue—contains thebaine, papaverine and narcotine
Step-4: Dissolve the residue or precipilate in dilute ethanol (50% v/v), make slightly acidic with the addition of dilute glacial acetic acid and finally add to it approximately three volumes of boiling distilled water.
Step-5: Filter the above reaction product:
Filtrate—contains thebaine
Residue—contains papaverine and narcotine
Step-6: Concentrate the filtrate obtained in Step-5 under reduced pressure and add to it dilute NH4OH solution to make it alkaline; and extract the liberated alkaloid thebaine successively with chloroform. Thebaine is obtained after evaportion of chloroform under vaccuo.
Characteristic Features
1. It is obtained as orthorhombic, rectangular plates by sublimation at 170-180°C under atmospheric pressure and a 1 mm distance mp 193°C (rapid heating).
2. Its physical parameters are: [α]D15 -2190(p = 2 in ethanol); [α]D23 (p = 5 in chloroform); pK at 15°C = 6.05; and pH of a saturated solution is 7.6.
3. Solubility Profile: 1 g dissolves in 1460 ml water at 15°C, in about 15 ml hot ethanol, 13 ml chloroform, 200 ml ether, 25 ml benzene, 12 ml pyridine; and not very soluble in petroleum ether.
Identification Tests Thebaine forms a number of salt derivatives which have specific characteristic features, such as:
1. Thebaine Salicylate (C19H21NO3.C7H6O3): It is obtained as crystals which are soluble in 750 parts of water. Thus, thebaine may be separated from other major alkaloids of opium by forming its salicylate derivative which is sparingly soluble in water.
2. Thebaine Hydrochloride Monohydrate (C19H21NO3.HCl.H2O): It is obtained as orthorhombic prisms from alcohol having [α]D23 -1640(p = 2). It is found to be soluble in about 12 parts of water and in ethanol. The pH of a 0.05 molar solution is 4.95.
3. Thebaine Oxalate Hexahydrate (2 C19H21NO3.C2H2O4.6 H2O): It is obtained as prisms. It is soluble in 10 parts of water and also in ethanol; and is almost insoluble in ether.
4. Thebaine Binoxalate Monohydrate (C19H21NO3.C2H2O4.H2O): It is obtained as prisms and found to be soluble in 45 parts of water.
5. Thebaine Bitartrate Monohydrate (C19H21NO3.C4H6O6.H2O): It is obtained as prisms, soluble in 130 parts of water, quite soluble in both hot water and hot ethanol.
6. It gives a red colour on the addition of a few drops of cold sulphuric acid which ultimately changes to orange yellow.
Uses It is an opiate analgesic.
D. Reticuline
Synonym Coclanoline.
Biological Sources It is obtained from the plant Hydratis canadensis L. (Ranunculaceae) (Goldenseal); the leaves of Laurus nobilis L. (Lauraceae) (Bay, Grecian Laurel, Green Bay); the air-dried milky exudation obtained from excised unripe fruits of Papaver somiferum L. (Papaveraceae) (Opium Poppy, Poppyseed Poppy Keshi); and the leaves of Sassafras albidum (Nutt.) Nees (Lauraceae) (Sassafras).
Chemical Structure
1, 2, 3, 4-Tetrahydro-1-[(3-hydroxy-4-methoxyphenyl) methyl]-6-methoxy-2-methyl-7-isoquinolinol; (C19H23NO4).
Isolation Gopinath et al.,* has described the isolation of d-form of reticuline from Anona reticulata Linn., (Annonaceae).
Characteristic Features
1. The dl-form of reticuline is obtained as pink crystals having mp 146°C.
2. The uvmax: 284 nm (log € 3.85).
3. Solubility Profile: It is soluble in aqueous buffer of pH < 7.5 or > 11; and is practically insoluble in water at pH 8-10.
Identification Tests
(S)-Form Reticuline Perchlorate (C19H23NO4.HClO4): It is obtained as colourless prisms from ethanol having mp 203-204°C. Its specific optical rotation [α]D18 +88.3o (C = 0.21 in ethanol).
-------------------------------------
* Gopinath et al., Ber. 92, 776 (1959).
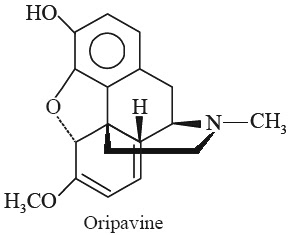
E. Oripavine
Synonym O3-Demethylthebaine.
Biological Sources It is obtained from the plant Papaver bracteatum Lindl. (Papaveraceae) (Great Scarlet Poppy, Thebaine Poppy); and Papaver orientale Linn. (Papaveraceae).
Chemical Structure
(5α)-6, 7, 8, 14-Tetrahydro-4, 5-epoxy-6-methoxy-17-methyl-morphinan-3-ol; (C18H19NO3).
Isolation It has been isolated from plant source by Kiselev and Konovalova.*
Characteristic Features The crystals have mp 200-201°C; and [α]D20 -211.80.
Identification Tests
1. Oripavine Hydrochloride (C18H19NO3.HCl): It is obtained as crystals which decompose at 244-245°C.
2. Oripavine Methiodide (C18H19NO3.CH3I): The crystals decompose at 207-208°C.
F. Morphinone It has been observed that the later stages of the biosynthetic pathway starting from reticuline leading to thebaine and morphine are strategically modified in some strains of opium poppy. Therefore, in such strains, thebaine is converted by way of oripavine and morphinone. In this pathway the phenolic O-methyl function is removed before that of the enol ether, i.e., accomplishing the same steps but in a different order. In other words, morphinone is obtained by the demethylation of oripavine as shown below:
Biosynthesis of Morphine, Codeine, Thebaine, Oripavine and Morphinone The various steps involved are as follows:
1. (R)-Reticuline, may be redrawn as shown in page 495 following pathway is found to be the substrate for one-electron oxidation via the phenol moiety present in each ring thereby yielding the diradical.
2. Subsequent coupling ortho to the phenol group in the tetrahydroisoquinoline nucleus, and para to the phenol in the benzyl substituent, gives rise to salutaridine—a dienone which is found as minor alkaloidal component in the opium poppy Papaver somniferum.
3. Thebaine is achieved via salutaridinol produced from salutaridine by means of the stereospecific reduction of the carbonyl group.
4. In thebaine the ring closure to form the ether linkage is caused due to the nucleophilic attack of the phenol moiety on the dienol system followed by a displacement of the hydroxyl group.
5. Future reactions essentially involve conversion of thebaine into morphine via codeine by virtue of a process that exclusively modifies the oxidation state of the diene ring, but apparently removes two O-methyl groups.
6. One is evidently present as an enol ether, removal of which yields neopinone, that subsequently gives rise to codeinone and then sodeine by the help of allylic isomerisation and reduction respectively.
7. In certain specific strains of opium poppy, thebaine is changed to oripavine and morphinone by virtue of the pathway that essentially removes the phenolic O-methyl function before that of the enol ether.
--------------------------------------------------------
* Kiselev and Konovalova J. Gen. Chem. USSR, 18, 142 (1948).